FIG 7.
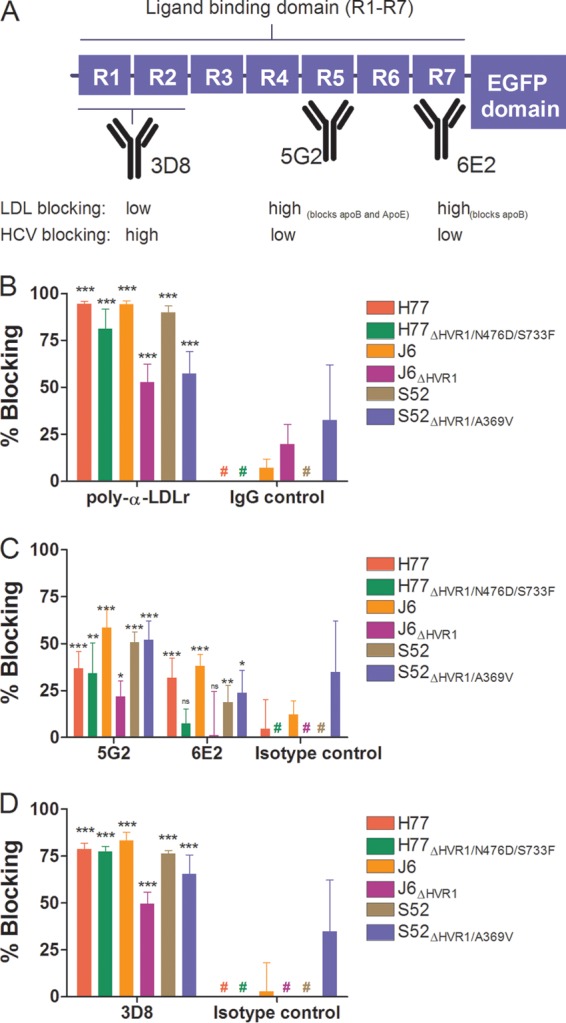
Monoclonal antibodies to three distinct regions of the LDLr ligand binding domain revealed that ApoB and ApoE are unlikely candidates for interaction between LDLr and HCV. (A) Schematic representation of LDLr with emphasis on the seven repeats (R1 to R7) of the ligand binding domain. The specificities of three MAbs are depicted, along with their abilities to decrease native LDL binding or HCV infection (cf. panels C and D). (B to D) Huh7.5 cells were plated in 96-well plates. The following day, the indicated LDLr blocking antibody (20 μg/ml of AF2148 [B], 50 μg/ml of 5G2 or 6E2 [C], or 50 μg/ml of 3D8 [D]) was added to the cells for 1 h at 37°C in 6 replicates, along with 6 replicates with only medium and 6 replicates with the relevant isotype controls (goat IgG, mouse IgG1, and mouse IgG2a). The indicated virus with and without HVR1 was added to the cells for 3 h of infection, and the cells were washed once with PBS and incubated for an additional 45 h in complete medium prior to HCV-specific staining. FFU counts at the given antibody concentrations were normalized to the mean FFU count of the 6 replicates of virus only. The data points are means of 6 replicates with SD. #, value below 0%. t tests were performed comparing the indicated LDLr-specific antibodies with virus only. *, **, and ***, statistical significance at P values of <0.05, 0.01, and 0.001, respectively; ns, not statistically significant.
