FIG 1.
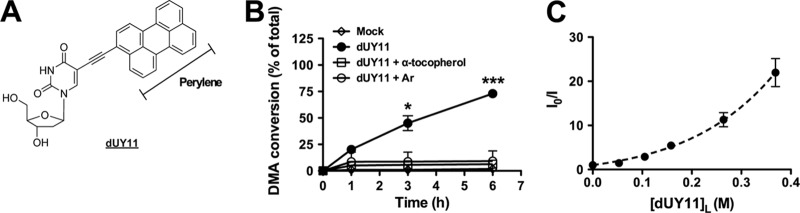
dUY11 generates singlet oxygen (1O2). (A) Structure of dUY11 and its perylene core. (B) dUY11 was added to a solution of 9,10-dimethylanthracene (DMA) and exposed to light. At 0.1, 1, 3, or 6 h, DMA conversion was detected by 1H-nuclear magnetic resonance (NMR) (DMA/oxiDMA = 3.1 ppm:2.1 ppm [methyl peak]). Reactions were performed in tetrahydrofuran (THF) using 1 equivalent of DMA and 0.5 equivalent of dUY11, and α-tocopherol where applicable, under an O2 atmosphere. Where applicable, THF was purged with argon (Ar) by the freeze-thaw method before the reaction was performed under Ar atmosphere. Mean ± standard deviation (SD), n = 2 independent measurements. *, P < 0.05; ***, P < 0.001, two-way analysis of variance (ANOVA) followed by a Bonferroni posttest for multiple comparisons. (C) DMA conversion by 1O2 production in 3 mM POPC (a C9 monounsaturated model phospholipid) LUV. It should be noted that concentrations indicated in POPC are the local concentration of dUY11 in the strict lipid bilayer volume, calculated as described in reference 25. 1O2 production was measured through its effect on a fluorescent 1O2 chemical trap. DMA reacts selectively with 1O2 in membranes to form the nonfluorescent 9,10-endoperoxide (DMAO2). By monitoring the disappearance of DMA's fluorescence signal (excitation at 379 nm and emission at 432 nm), we were able to estimate the level of 1O2 in the membrane. 1O2-associated fluorescence disappearance data were analyzed using the quenching sphere-of-action model, , where is the apparent Stern-Volmer constant (indicating the reduction of fluorescence by the conversion of DMA to DMAO2), V is the sphere-of-action volume (i.e., the sphere that surrounds the chromophore within which the “quencher” can be considered to be in contact with the chromophore), and NA is Avogadro's constant. Mean ± SD, n = 3 independent measurements.
