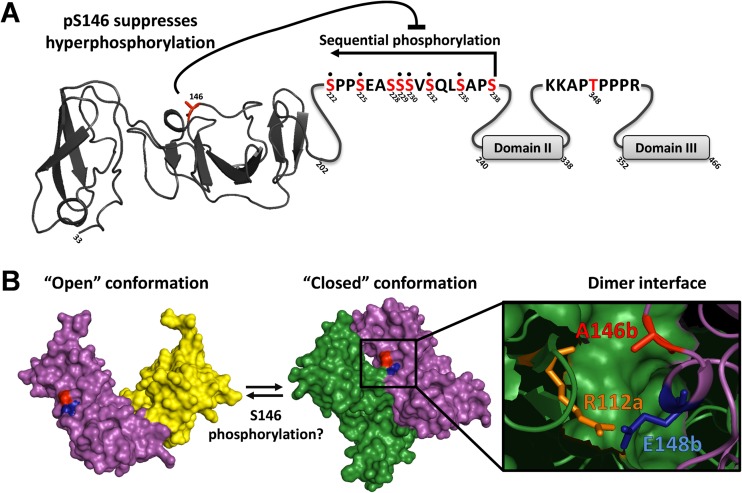
FIG 9.
Summary and potential role of NS5A phosphorylation. (A) Schematic of NS5A showing the location and relationship of phosphorylation events identified. Domain I is denoted by the structure of the Con1 monomer (6); sequences of LCS I and LCS II are shown; domains II and III are denoted by gray boxes. Highlighted in red are those residues identified as phosphorylation sites. Note that residues S228 and S238 were not observed directly by mass spectrometry, but the presence of a heptaphosphorylated phosphopeptide with only 8 possible phosphorylation sites strongly indicates their presence. (B) The two differing dimeric conformations of NS5A domain I are shown, “closed” (3FQM) (5) and “open” (1ZH1) (6), green/purple and purple/yellow, respectively. The structural studies utilized a Con1 sequence where an alanine is present at position 146. Residue E148b (conserved in JFH-1 and Con1) forms an intramolecular bond with R112a on the opposite monomer, blue and orange, respectively (5). It is likely that phosphorylation at 146 would exert an effect on this key dimer interaction of the “closed” conformation.