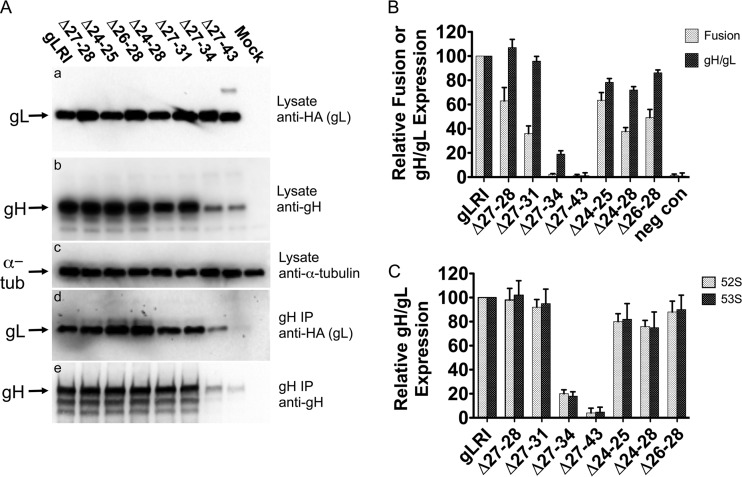
FIG 2.
HSV-1 gH/gL deletion mutant expression, coimmunoprecipitation, and function in cell-cell fusion. (A) Cells were transfected with plasmids expressing genes for gH and gLRI or one of the deletion mutants. After 48 h, the cells were processed for Western blotting with the following antibodies: (a) an anti-HA monoclonal antibody to detect wild-type or mutant gL expression; (b) R137 rabbit antiserum to detect gH expression; (c) anti-alpha-tubulin monoclonal antibody to monitor alpha-tubulin expression as a control. In parallel, the lysates from the transfected cells listed above were immunoprecipitated (IP) with anti-gH monoclonal antibody 52S, and the resulting immunoprecipitates were subjected to Western blotting with the following antibodies: (d) anti-HA monoclonal antibody to detect wild-type or mutant gL associated with gH; (e) R137 rabbit antiserum to detect levels of immunoprecipitated gH. Antibody binding to blots was detected using chemiluminescence. Mock cells were transfected with a plasmid expressing a negative control protein, angiotensin-converting enzyme 2. This experiment was performed three independent times, and the results of one representative replicate are shown. (B) Cell-cell fusion and gH/gL cell surface expression for gL mutants. CHO cells expressing HSV-1 gB, gD, or gH or T7 polymerase and either gLRI, a gL deletion mutant, or pcDNA3 (negative control [neg con]) were mixed with CHO cells expressing nectin-1 and the T7 polymerase-inducible β-Gal. The microgram ratio, gB:gD:gH:gL:T7, for transfected expression plasmids was 1:1:1:0.13:1. The β-Gal activity was measured as an indication of cell-cell fusion. Cell surface gH/gL expression was measured in envelope glycoprotein-expressing cells via CELISA with the anti-gH 52S monoclonal antibody. Mean relative values and mean standard deviations from at least three independent experiments are shown. Within each experiment, values are expressed as the percentage of the results of the transfection expressing gLRI. There was a significant difference between the mean cell fusion activity for gLRI and Δ27-28 (P = 0.015) and for gLRI and Δ27-31 (P = 0.0009), as determined by using a two-tailed unpaired t test. (C) Monoclonal antibody binding to gH in the presence of gL or one of the deletion mutants. CHO cells expressing gH and either gLRI or a deletion mutant were analyzed for cell surface gH/gL expression via CELISA. Two anti-gH monoclonal antibodies were used in parallel, 52S or 53S. Mean relative values and mean standard deviations from at least three independent experiments are shown. For each experiment, values are expressed as a percentage of the transfection expressing gLRI.