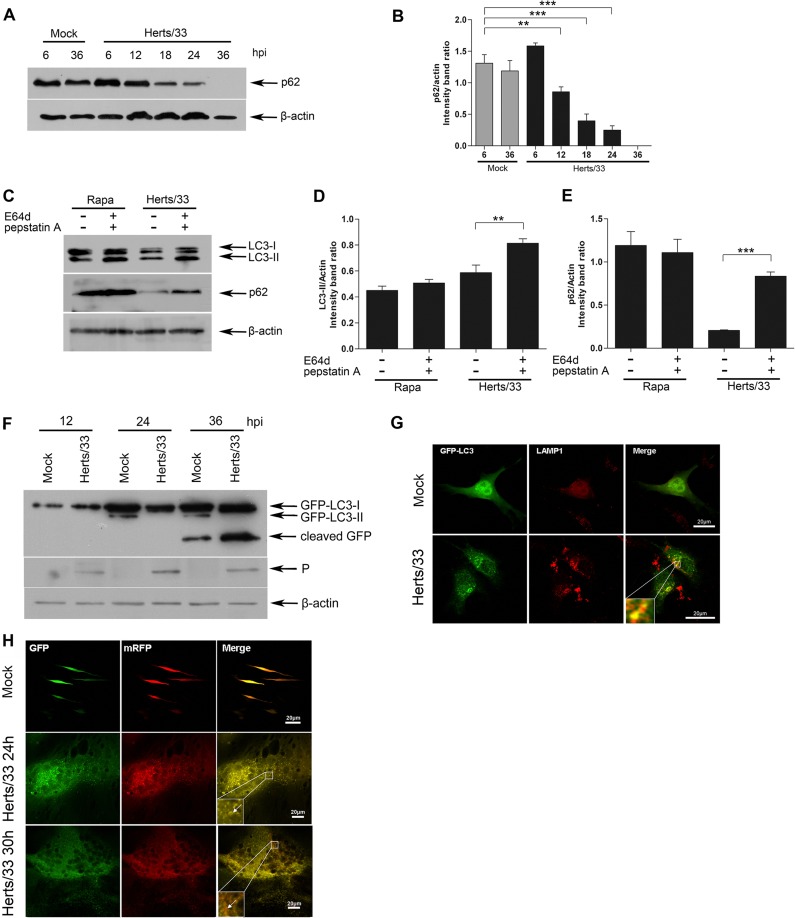
FIG 2.
Measurement of the autophagic flux in DF-1 cells infected with NDV. (A) Western blotting of the degradation of p62. p62 in DF-1 cells infected with NDV Herts/33 at an MOI of 1 was monitored using anti-p62 antibody. Mock-infected DF-1 cells were used as negative controls. β-Actin was used as a protein loading control. (B) Representative results are shown with graphs representing the ratio of LC3-II to β-actin normalized to the control condition. Data are presented as means from three independent experiments. Significance is analyzed with two-tailed Student's t test (**, P ≤ 0.01; ***, P ≤ 0.001), compared to the control group. (C) DF-1 cells were subjected to a 1-h absorption period of NDV Herts/33 and further cultured in fresh medium in the absence (−) or presence (+) of E64d and pepstatin A at 10 μg/ml each. Western blotting was performed using anti-LC3B and anti-p62 antibodies. Rapamycin-treated DF-1 cells were used as controls. β-Actin was used as a protein loading control. (D and E) Representative results are shown with graphs representing the ratio of LC3-II to β-actin (D) or p62 to β-actin (E) normalized to the control condition. Data are presented as means from three independent experiments. Significance is analyzed with two-tailed Student's t test (**, P ≤ 0.01; ***, P ≤ 0.001), compared to the control group. (F) DF-1 cells were transfected with GFP-LC3 followed by treatment at 24 h posttransfection with infection of NDV Herts/33 at an MOI of 1 or mock treatment as a negative control. Cells were harvested at indicated time points and detected with anti-GFP and anti-P antibody. β-Actin was used as a protein loading control. (G) DF-1 cells transfected with GFP-LC3 were mock infected or infected with NDV Herts/33 at an MOI of 1. Cells were fixed and stained with anti-LAMP1 antibody and then visualized by confocal microscopy. Bars, 20 μm. (H) DF-1 cells transfected with ptfLC3 were mock infected or infected with NDV Herts/33 at an MOI of 1. The cells were collected, fixed, and visualized at 24 h and 30 h postinfection, respectively. Bars, 20 μm.