FIG 2.
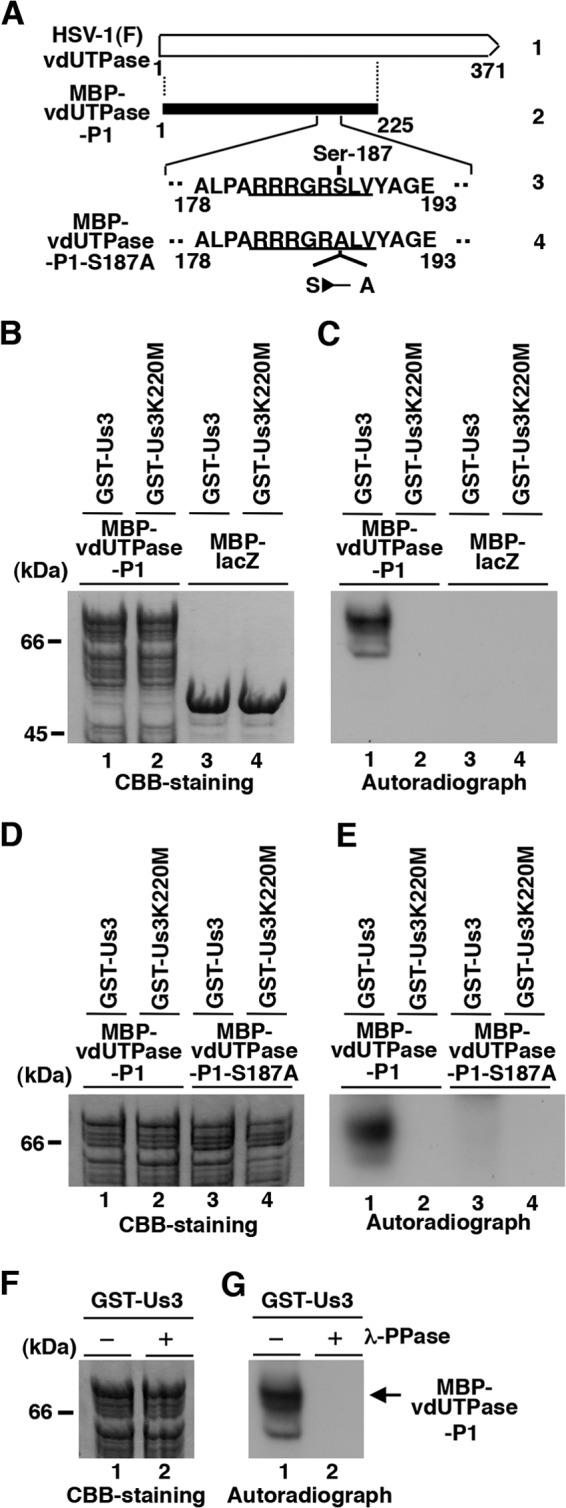
Us3 directly phosphorylated vdUTPase Ser-187 in vitro. (A) Schematic diagram of vdUTPase. Line 1, structure of the vdUTPase open reading frame. Line 2, domain of the vdUTPase gene encoding vdUTPase residues 1 to 225, which were used in these studies to generate the MBP-vdUTPase-P1 fusion protein. Line 3, amino acid sequence of vdUTPase residues 178 to 193. The site with the consensus sequence for phosphorylation by Us3 is underlined. Line 4, domain of the UL50 (vdUTPase) gene encoding vdUTPase residues 178 to 193 with the S187A mutation used in these studies to generate the MBP-vdUTPase-P1-S187A fusion protein. (B) Purified MBP-vdUTPase-P1 (lanes 1 and 2) and MBP-LacZ (lanes 3 and 4) incubated in kinase buffer containing [γ-32P]ATP and purified GST-Us3 (lanes 1 and 3) or GST-Us3K220M (lanes 2 and 4), separated on an SDS-PAGE gel, and stained with Coomassie brilliant blue (CBB). (C) Autoradiograph of the gel in panel B. (D) Purified MBP-vdUTPase-P1 (lanes 1 and 2) and MBP-vdUTPase-P1-S187A (lanes 3 and 4) incubated in kinase buffer containing [γ-32P]ATP and purified GST-Us3 (lanes 1 and 3) or GST-Us3K220M (lanes 2 and 4), separated on an SDS-PAGE gel, and stained with CBB. (E) Autoradiograph of the gel in panel D. (F) Purified MBP-vdUTPase-P1 incubated in kinase buffer containing [γ-32P]ATP and purified GST-Us3 and then either mock treated (lane 1) or treated with λ-PPase (lane 2), separated on an SDS-PAGE gel, and stained with CBB. (G) Autoradiograph of the gel in panel F. A molecular mass marker (in kilodaltons) is shown on the left.
