FIG 6.
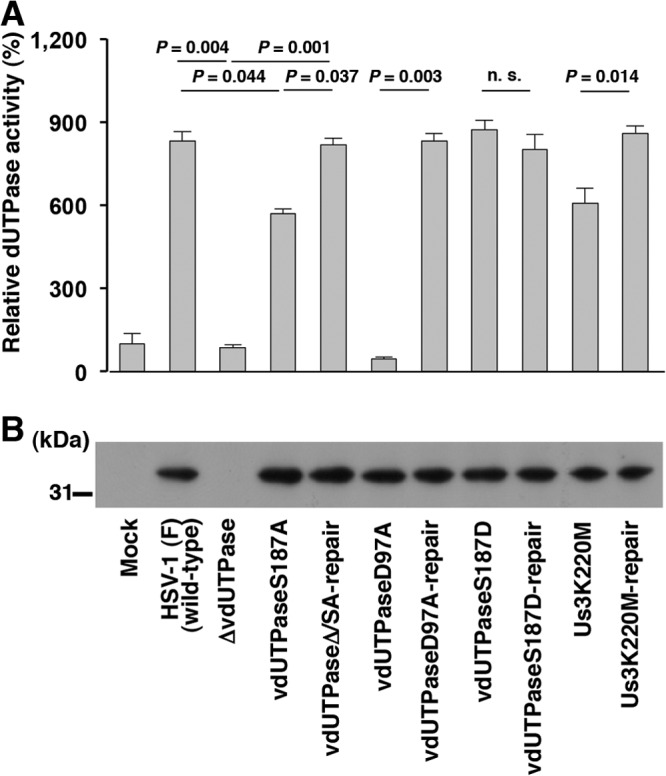
Us3-mediated phosphorylation of vdUTPase Ser-187 upregulated its enzymatic activity in infected cells. (A) Vero cells were mock infected or infected with wild-type HSV-1(F), YK750 (ΔvdUTPase), YK751 (vdUTPaseS187A), YK752 (vdUTPaseΔ/SA-repair), YK759 (vdUTPaseD97A), YK760 (vdUTPaseD97A-repair), YK753 (vdUTPaseS187D), YK754 (vdUTPaseS187D-repair), YK511 (Us3K220M), or YK513 (Us3K220M-repair) at an MOI of 5. At 18 h postinfection, the cells were solubilized and assayed for dUTPase activity. Each value is the mean ± standard error of the results of triplicate experiments and is expressed relative to the mean value of dUTPase activity in mock-infected cells, which was normalized to 100%. “P” represents the statistical significance value according to the two-tailed Student t test. n.s., not significant. Data are representative of three independent experiments. (B) Immunoblot of lysates prepared for the assays in panel A. The cell lysates were analyzed by immunoblotting with anti-vdUTPase mouse polyclonal antibody. A molecular mass marker (in kilodaltons) is shown on the left. Data are representative of three independent experiments.
