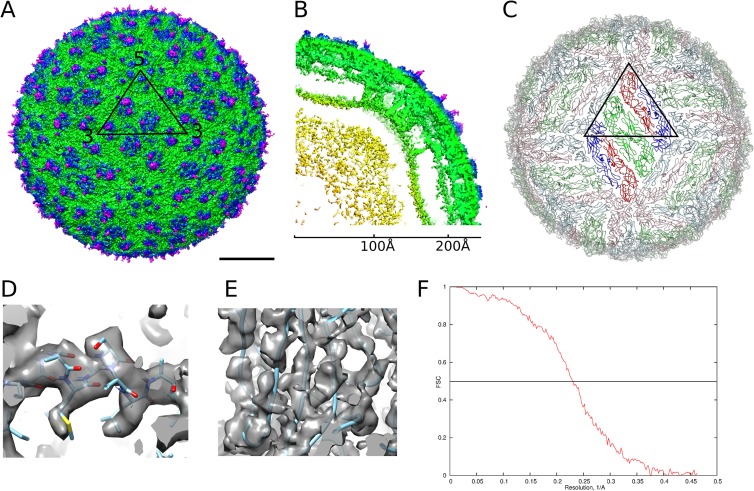
FIG 1.
Cryo-EM structure of DENV4 solved to 4.1-Å resolution. (A) Surface of the DENV4 cryo-EM map. The black triangle shows the icosahedral asymmetric unit. Scale bar, 100 Å. (B) A quarter of a slice through the center, showing radial density distribution. The map is colored radially in panels A and B: up to 160 Å, yellow; 161 Å to 220 Å, green; 221 Å to 230 Å, blue; from 230 Å, magenta. (C) A view of the herringbone arrangement of the E proteins. The three individual E proteins in each asymmetric unit are colored in red, green, and blue. The E proteins in the raft in the center, which consist of two asymmetric units, are in brighter colors. (D and E) Views of the electron density map fitted with structures of the helical E stem region (D), showing densities corresponding to bulky side chains, and β-sheets (E), showing that the densities of β-strands are well separated. (F) A plot of Fourier shell correlation.