Table 1.
Guidelines for proper use of fluorescent probes.
| Fluorogenic probe | Advantages | Disadvantages | Recommendations |
|---|---|---|---|
HE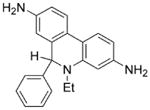
|
Reacts with
to form a diagnostic marker product, 2-OH-E+. Reacts with other oxidants (e.g., •OH, ONOO−) to form E+ and dimers. Intermediate HE-derived radical does not react with molecular oxygen to form and H2O2. |
2-OH-E+ and E+ have similar fluorescence spectral characteristics. Further extraction and HPLC analyses are needed for proper identification. |
Suitable for detection of intracellular
. Suitable for detection of intracellular oxidants (e.g., iron and H2O2, cytochrome c and H2O2). However, red fluorescence measurements using fluorescence or confocal microscopy will not be able to distinguish between E+ and 2-OH-E+. HPLC or other analytical measurements of products are crucial. |
Mito-SOX or Mito-HE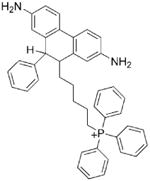
|
Localizes into mitochondria. Reacts with to form a diagnostic marker product, 2-OH-Mito-E+. Reacts with other oxidants (•OH, ONOO−) to form Mito-E+ and dimers. Intermediate Mito-HE-derived radical does not react with O2 to form and H2O2. |
2-OH-Mito-E+ and Mito-E+ have similar fluorescence spectral parameters. HPLC analyses of products are necessary for proper identification probe. Mitochondrial toxicity is a concern. |
Suitable for detection of mitochondrial
. Suitable for detection of intracellular oxidants (e.g., iron and H2O2, cytochrome c and H2O2). However, the “red fluorescence” measurements alone will not allow distinction between the hydroxylated and the oxidized Mito-HE. HPLC or other analytical measurements of products are crucial. |
DCFH-DA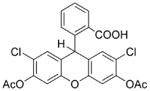
|
Cell-permeable Easy to use. Responds to changes in intracellular iron signaling or enhanced peroxidase activity (see Ref. [36]). |
Artifactual amplification of the fluorescence intensity via a redox-cycling mechanism involving an intermediate radical, DCF•−. | Not suitable for measuring intracellular H2O2 or other oxidants. May be used as a redox indicator probe for uncovering new redox signaling mechanisms keeping in mind the various caveats. |
DHR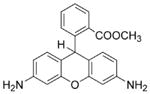
|
Cell-permeable Easy to use. Responds to cogenerated •NO and via a predictable radical chemistry. |
Artifactual amplification of the fluorescence intensity via a redox-cycling mechanism involving an intermediate radical, DHR•. | Not suitable for measuring intracellular ONOO− or other oxidants. May be used as a nonspecific indicator for intracellular ONOO− or oxidants derived from it. |
Coumarin boronate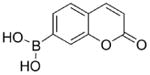
|
Reacts very rapidly and nearly stoichiometrically with ONOO− to form a fluorescent product. | Further metabolism of the product (7-hydroxycoumarin) and possible excretion out of cells may be a hindrance. | Suitable for measuring extracellular ONOO− formation. As this probe also reacts with H2O2 (albeit slowly), proper controls using appropriate inhibitors should be performed. |
Amplex red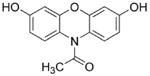
|
HRP/H2O2-dependent oxidation of Amplex red to resorufin is very efficient. The intermediate Amplex red-derived radical does not react with O2 for form additional and H2O2. The intermediate Amplex red-derived radical will react with to decrease resorufin formation (see Ref. [37]). Reducing agents (e.g., ascorbate) and peroxidase substrates (nitrite anion) will inhibit resorufin formation. |
Room light-mediated photochemical oxidation of resorufin in the presence of GSH, NAD(P)H, or ascorbate will greatly increase artifactual generation of H2O2. Amplex red-derived radical can rapidly react with and inhibit product formation. |
Suitable for measuring extracellular H2O2 under conditions limiting the secondary radical reactions induced by resorufin. |
