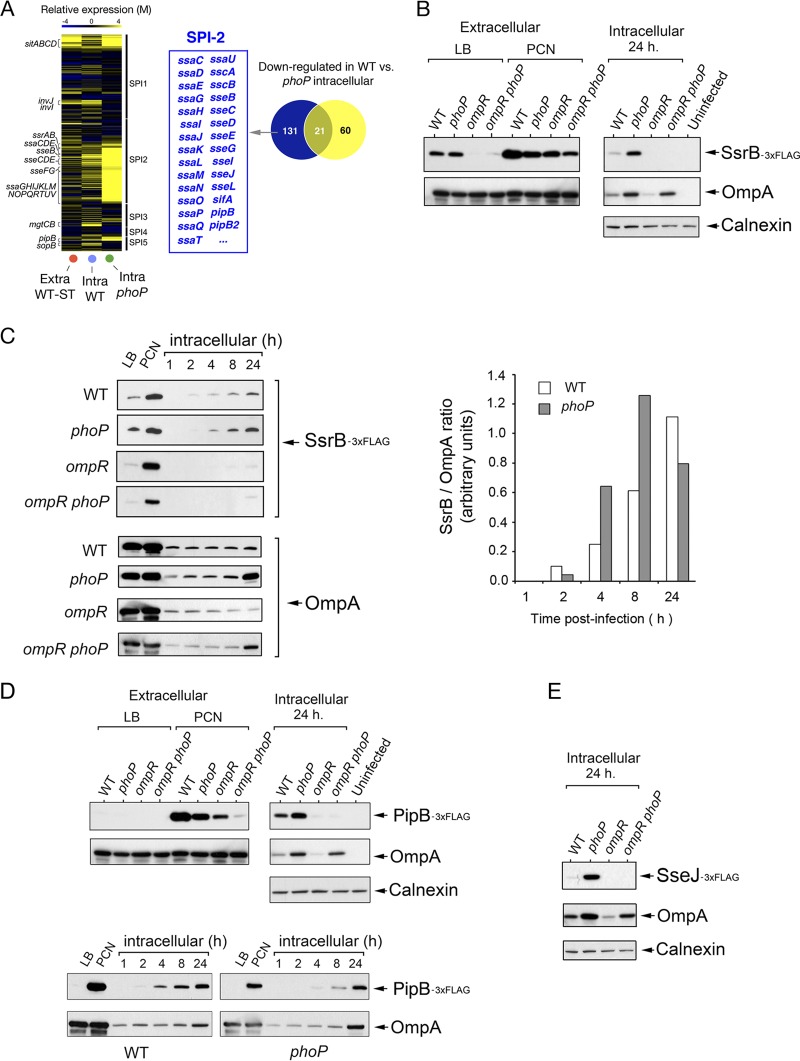
FIG 1.
Distinct contribution of PhoP-PhoQ and OmpR-EnvZ to SPI-2 regulation in dormant intracellular S. Typhimurium. (A) Heat map showing the expression prolife of S. Typhimurium pathogenicity island genes (SP-1, SPI-2, SPI-3, SPI-4, and SPI-5) in nongrowing (wild-type) and overgrowing bacteria (phoP mutant) at 24 h postinfection of NRK-49F fibroblasts. As reference, the expression profile of extracellular wild-type bacteria grown to stationary phase (Extra-WT-ST) is shown. The Venn diagram indicates genes that are expressed at higher levels in intracellular phoP bacteria than in nongrowing dormant wild-type bacteria, including genes of the SPI-2 regulon. (B) Levels of the SPI-2 regulator SsrB in isogenic strains with an ssrB::3×FLAG chromosomal allele and differences in the functionality of the PhoP-PhoQ and OmpR-EnvZ two-component systems. The analysis was performed in extracellular bacteria grown in rich (LB) or chemically defined (PCN) nutrient media, and intracellular bacteria were collected at 24 h postinfection. (C) Kinetics revealing the postinfection time (∼2 h) at which intracellular dormant bacteria initiate the production of SsrB. Note the delay and reduction in the production of SsrB levels displayed by phoP and ompR mutants, respectively. Such delay was also observed upon quantification by densitometry of the relative levels of SsrB with respect to the internal control, OmpA. (D) Expression pattern of the SPI-2 effector protein PipB in extracellular bacteria grown in LB and PCN media and in intracellular bacteria at 24 h postinfection of NRK-49F fibroblasts. Note the correspondence between these data and those obtained for the SPI-2 dedicated regulator SsrB. Kinetics analysis performed in parallel also showed such similarities. (E) Relative levels of the SPI-2 effector SseJ in intracellular bacteria collected at 24 h postinfection of NRK-49F fibroblasts. The expression prolife matches those of SsrB and PipB. OmpA and calnexin were used as loading controls.