FIG 7.
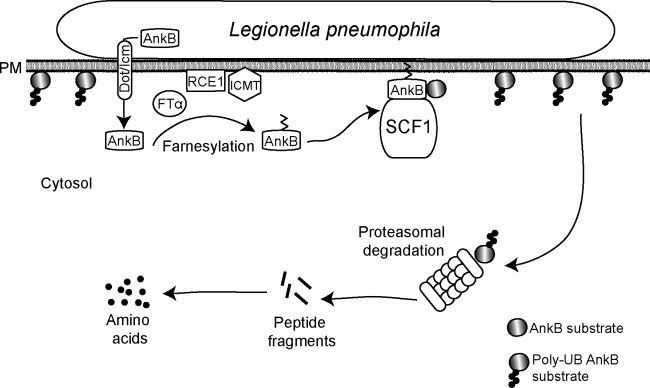
Working model of AnkB-mediated nutritional preparation of the host cell by attached extracellular L. pneumophila. Upon bacterial attachment, the Dot/Icm translocation system is triggered to inject bacterial effectors, one of which is AnkB. The host farnesylation enzymes are recruited beneath bacterial attachment sites and are essential for farnesylation of AnkB, which enables anchoring of this effector to the cytosolic side of the plasma membrane beneath bacterial attachment sites. The host SCF1 ubiquitin ligase complex is recruited to the plasma membrane, where it interacts with the F-box domain of AnkB to trigger assembly of K48-linked polyubiquitinated proteins beneath bacterial attachment sites. Proteasomal degradation of the K48-linked polyubiquitinated proteins generates higher levels of cellular amino acids, particularly the limiting ones, such as cysteine, which is a metabolically preferable source of carbon and energy for L. pneumophila. The availability of higher levels of cell amino acids upon entry of L. pneumophila circumvents a potential starvation response and differentiation into the motile nonreplicative phase for the entering bacterium. The elevated cellular levels of amino acids trigger differentiation of L. pneumophila into the replicative phase and are main sources of carbon and energy that feed the TCA cycle to power intracellular bacterial proliferation.
