FIG 6.
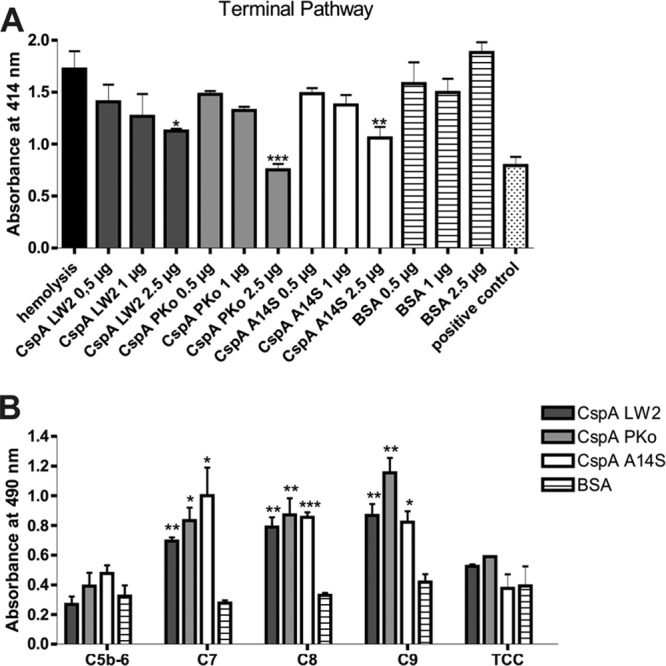
CspA orthologs inhibit TCC formation by interaction with complement components of the terminal pathway. (A) Inhibition of the TP was investigated using SE preincubated with C5b-6 employing hemolytic assays. A reaction mixture containing C7, C8, and C9 was preincubated with increasing concentrations of CspA orthologs or BSA. Following incubation, erythrocyte lysis was detected at 414 nm. Means from three separate experiments are shown, and error bars correspond to SD. Raw data were analyzed using one-way ANOVA with a post hoc Bonferroni correction. **, P < 0.01, *, P < 0.05. (B) Binding of complement components was verified for CspA orthologs using ELISA. Hexahistidine-tagged recombinant proteins or BSA (5 μg/ml each) was immobilized onto microtiter plates and incubated with purified C5b-6, C7, C8, C9, or TCC. Bound complement components were detected using specific antibodies and HRP-conjugated anti-goat immunoglobulins. Absorbance was measured at 490/560 nm. Experiments were performed in triplicate, and graphs represent means from at least three independent experiments. Error bars indicate standard errors of the means. ***, P < 0.001; **, P < 0.01; *, P < 0.05 (Student t test).
