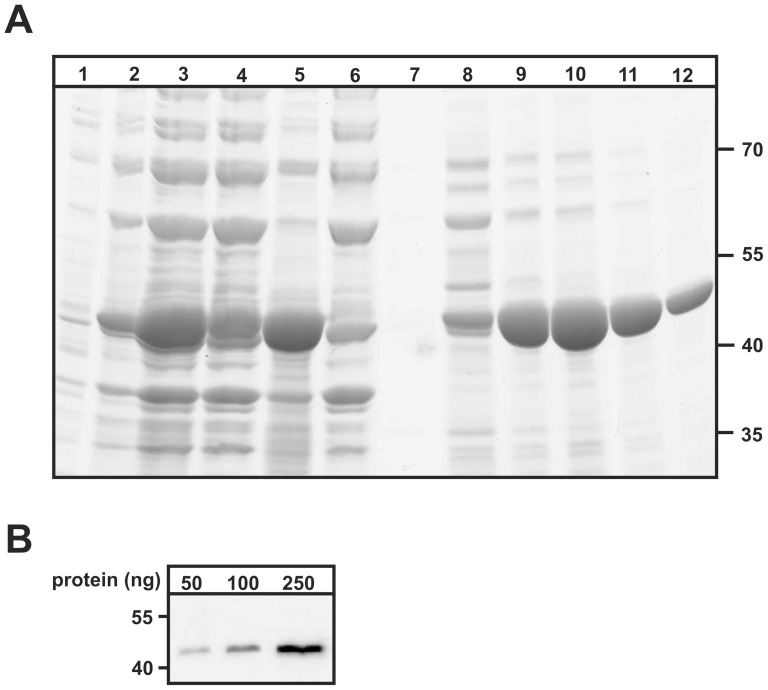
Figure 1. Ni-NTA purification of GCDH His6-fusion protein.
(A) The expression of recombinant GCDH-His6 in E. coli was induced by the addition of IPTG (lane 1: before induction, lane 2: after induction). After 4 h E. coli cells were lysed by sonication (lane 3) and centrifuged (lane 4: pellet with insoluble proteins; lane 5: supernatant with soluble proteins). The supernatant with GCDH-His6 was incubated with Ni-NTA agarose and loaded on a column. Unbounded proteins (lane 6) were removed and the column was washed with increasing imidazole concentrations (lane 7: 10 mM imidazole; lane 8: 50 mM imidazole). Finally the GCDH-His6 protein was eluted in four steps with increasing imidazole concentrations (lane 9–11: 150 mM imidazole; lane 12: 250 mM imidazole). Samples were separated by SDS-PAGE (10% acrylamide) and proteins were visualized by Coomassie Blue staining. The positions of molecular mass marker proteins (in kDa) are indicated. (B) Validation of the purified GCDH-His6-fusion protein. Different amounts of purified GCDH-His6 protein were separated by SDS-PAGE (10% acrylamide) and analyzed by anti-GCDH western blotting. Representative pictures of n = 10 independent preparations are shown.