Abstract
Objective
Examine disparities in use of cardioprotective medications in treatment of peripheral artery disease (PAD) by socioeconomic status (SES).
Background
PAD is associated with increased cardiovascular risk and is more prevalent among those of lower SES. However, the use of guideline-recommended secondary preventive measures for the treatment of PAD across diverse income subgroups and the influence of practice site on potential treatment disparities by SES are unknown.
Methods
Within the National Cardiovascular Disease Registry (NCDR®) PINNACLE Registry®, 62,690 patients with PAD were categorized into quintiles of SES, as defined by the median income of each patient’s zip code. The association between SES and secondary prevention treatment with antiplatelet and statin medications was evaluated using sequential hierarchical modified Poison models, adjusting first for practice site and then for clinical variables.
Results
Compared with the highest SES quintile (median income >$60,868), PAD patients in the lowest SES quintile (median income <$34,486) were treated less often with statins (72.5 % vs. 85.8%; RR 0.84 [0.83–0.86]; P<0.001) and antiplatelet therapy (79.0% vs. 84.6 %; RR 0.93 [95% CI: 0.91–0.94]; P<0.001). These differences were markedly attenuated after controlling for practice site variation: statins (adjusted RR: 0.97 [0.95–0.99]; P=0.003) and antiplatelet therapy (adjusted RR 0.98 [0.97–1.00]; P=0.012). Additional adjustment for patients’ clinical characteristics had minimal impact with slight further attenuation: statins (adjusted RR: 1.00 [0.99–1.01]; p=0.772) and antiplatelet therapy (adjusted RR: 1.00 [0.99–1.01]; p=0.878).
Conclusion
Among PAD patients, the practice site at which patients received care largely explained the observed SES differences in treatment with guidelines-recommended secondary prevention medications. Future efforts to reduce treatment disparities in these vulnerable populations should target systems improvement at practices serving high proportions of patients with low SES.
Background
The Institute of Medicine has challenged the US health care system to minimize disparities in treatment and provide equitable access to evidence-based therapies to all patients1. While there have been numerous studies investigating disparities in the care of cardiac patients,2–4 disparities research in peripheral arterial disease (PAD) has been limited,5–9 even though PAD affects over 7 million Americans10 and disproportionately affects those of lower socioeconomic status (SES). 11,12 Equitable access to inexpensive guideline-recommended secondary preventive therapies has the potential to improve cardiovascular outcomes in this vulnerable population, but whether treatment rates differ across income groups remains unknown. Given that PAD is associated with increased cardiovascular risk and mortality18–22, illuminating current practice patterns by SES for evidence-based secondary preventive strategies is particularly important in defining opportunities to better improve care.
Accordingly, we examined PAD treatment rates by SES within the American College of Cardiology’s National Cardiovascular Disease Registry (NCDR®) PINNACLE Registry®, which prospectively captures information on the clinical care of outpatients, including the use of guideline-recommended secondary preventive therapies. Given the potential variability in care across clinics, we explicitly sought to examine both variations in secondary prevention treatment of PAD by SES and whether treatment differences by SES were explained at the site level, with the hope that our findings could not only identify potential disparities by income, but also define targets for future interventions to reduce disparities in PAD care.
Methods
Study Population
The PINNACLE Registry was launched in 2008 and represents the first national, prospective, office-based, quality improvement registry of cardiovascular patients in the United States.23,24 Among participating practices, patient data were collected at the point of care for a variety of cardiovascular conditions, including coronary artery disease, heart failure, atrial fibrillation and PAD. Participation in this quality improvement initiative is voluntary.
For the purposes of this study, we identified 66,282 patients with a diagnosis of PAD enrolled from 61 practices between July 1, 2010, and June 30, 2011. Within the PINNACLE Registry, PAD was defined by one of the following self-identified criteria by the patients: 1) claudication, either with exertion or at rest; 2) amputation for arterial vascular insufficiency; 3) vascular reconstruction, bypass surgery, or percutaneous intervention to the extremities (excluding dialysis fistulas and vein stripping); 4) documented aortic aneurysm with or without repair; or 5) positive non-invasive test (e.g., ankle brachial index <=0.9, ultrasound, magnetic resonance, computed tomography) or diagnostic angiographic stenosis of > 50% in any major peripheral artery (e.g., renal, subclavian, femoral, iliac). We excluded 2,945 patients for whom information on SES was missing (zip code data was not available). Our final study cohort was comprised of 62,690 PAD patients from 61 sites. For the analyses in this study, as patients may have had multiple visits in the PINNACLE Registry, we used information from the first visit to represent each patient only once.
SES and Processes of Care
The key independent variable was patients’ SES, which was defined by the median income of the patient’s zip code of residence. This approach to categorize levels of socioeconomic status has been used in previous reports of various disease conditions.25–27 The primary study outcome was treatment with 2 secondary prevention medication therapies: antiplatelet therapy (aspirin or clopidogrel) and statins, which are both Class I indications for PAD by the ACC/AHA PAD guidelines and PAD performance measures.28,29 Patients with documented contraindications to antiplatelet therapy (e.g., history of gastrointestinal bleeding) or statins were excluded within the analysis for each treatment. Moreover, for the analyses with antiplatelet therapy, we further excluded 9,295 patients already on warfarin therapy given that warfarin may influence use of antiplatelet therapy.
Statistical Analysis
Patients were categorized into quintiles of SES, with Quintile 1 representing the lowest SES and Quintile 5 the highest. Baseline differences across quintiles of SES were then compared using Mantel-Haenszel trend test for categorical variables and linear trend test for continuous variables.
Separate multivariable hierarchical modified Poison models were used to assess the relationship between SES and treatment with antiplatelet therapy and statins. We employed 2-level hierarchical models to adjust for clustering of patients within practices, with individual practices modeled as random effects and other patient characteristics modeled as fixed effects within each practice30. This approach allowed us to control for measured and unmeasured between-practice confounding, as the use of hierarchical models ensured that patients with similar SES were compared with each other from the same practice.
To better understand the extent of the practice site variation in accounting for treatment differences by SES, we performed a 2-step sequential adjustment. First, we adjusted for practice site only to assess the extent to which differences by SES were attenuated. This step allows us to understand whether treatment differences by SES persist when comparing patients of different SES within the same site. Next, we additionally controlled for clinical characteristics, including age, gender, insurance status, diabetes, dyslipidemia, history of MI, history of revascularization in the past 12 months, history of congestive heart failure, and history of stroke.
For each analysis, the null hypothesis was evaluated at a two-sided significance level of 0.05, with 95% confidence intervals (CIs) calculated using robust standard errors. All analyses were performed with SAS 9.3 (SAS Institute, Cary, NC) and R version 2.10.0.
Results
Baseline characteristics of the 62,690 PAD patients by SES quintiles are summarized in Table 1. Patients in the lowest SES quintile were from zip codes with median household incomes of less than $34,486 annually, while those in the highest quintiles were from zip codes with annual median household incomes of greater than $60,868.
Table 1.
Baseline demographics of the study cohort, stratified by socioeconomic status
| Quintiles of Socioeconomic Status | Total | P-Value | |||||
|---|---|---|---|---|---|---|---|
| Quintile 1 n = 12521 |
Quintile 2 n = 12513 |
Quintile 3 n = 12565 |
Quintile 4 n = 12511 |
Quintile 5 n = 12580 |
n = 62690 | ||
| Median Income (IQR) | 4,583–34,486 | 34,486–41,117 | 41,118–50,371 | 50,372–60,868 | 60,869–200,001 | <0.001 | |
| Age (Median (IQR)) | 69.0 (61.0, 78.0) | 71.0 (62.0, 79.0) | 70.0 (62.0, 78.0) | 71.0 (63.0, 79.0) | 71.0 (63.0, 79.0) | 70.0 (62.0, 78.0) | < 0.001 |
| Male | 60.6 | 62.8 | 62.5 | 61.7 | 64.2 | 62.4 | < 0.001 |
| Insurance Category | |||||||
| Private Insurance | 54.5 | 63.3 | 59.7 | 58.7 | 61.0 | 59.4 | < 0.001 |
| Public Insurance | 41.1 | 33.0 | 37.2 | 37.9 | 35.2 | 36.9 | <0.001 |
| No Insurance | 4.5 | 3.7 | 3.1 | 3.4 | 3.8 | 3.7 | <0.001 |
| Comorbidities | |||||||
| Coronary artery disease | 86.9 | 86.2 | 86.6 | 83.6 | 83.5 | 85.4 | < 0.001 |
| Dyslipidemia | 77.2 | 80.8 | 82.1 | 82.7 | 84.5 | 81.5 | < 0.001 |
| Diabetes | 33.4 | 34.0 | 32.2 | 31.5 | 32.2 | 32.7 | < 0.001 |
| Hypertension | 82.3 | 85.2 | 84.4 | 82.3 | 82.4 | 83.3 | 0.010 |
| Prior stroke or TIA | 11.5 | 24.1 | 25.2 | 16.4 | 18.0 | 19.0 | < 0.001 |
| Heart failure | 45.4 | 53.6 | 50.9 | 40.1 | 36.5 | 45.3 | < 0.001 |
| Myocardial infarction history | 30.9 | 29.9 | 31.8 | 28.7 | 31.5 | 30.6 | 0.982 |
| CABG within 12 months | 23.29 | 25.4 | 33.8 | 33.2 | 35.8 | 30.3 | < 0.001 |
| Percutaneous coronary intervention within 12 months | 47.0 | 48.8 | 43.0 | 33.7 | 34.7 | 41.4 | < 0.001 |
Continuous variables compared using linear trend test.
Categorical variables compared using Mantel-Haenszel trend test.
Defined by patient’s zip code level annual median household income
Public insurance refers to Medicare, Medicaid, military, and state insurance
The median age of the overall cohort was 70.0 (IQR 62.0, 78.0) years and 62.4% were men. Nearly 60% of patients had private insurance, and only 4.2% were uninsured. There was a high prevalence of coronary artery disease (85.4%), dyslipidemia (81.5%), and hypertension (83.3%) in the cohort. Nearly one-third of patients were diabetic and one-quarter were active smokers. Finally, 30.3% of patients had undergone coronary artery bypass surgery whereas 41.4% had undergone percutaneous coronary intervention within the past year.
Compared with patients in the highest SES quintiles, patients in the lower SES quintiles were slightly younger, more frequently female, and less likely to have private health insurance. Patients in lower SES quintiles were also more likely to have undergone percutaneous coronary intervention in the past year and be active smokers, and were less likely to have undergone coronary artery bypass surgery in the past year or have had a prior stroke. Lastly, rates of dyslipidemia, diabetes, hypertension, congestive heart failure, and prior myocardial infarction were clinically similar across quintiles.
Use of Cardioprotective Therapy
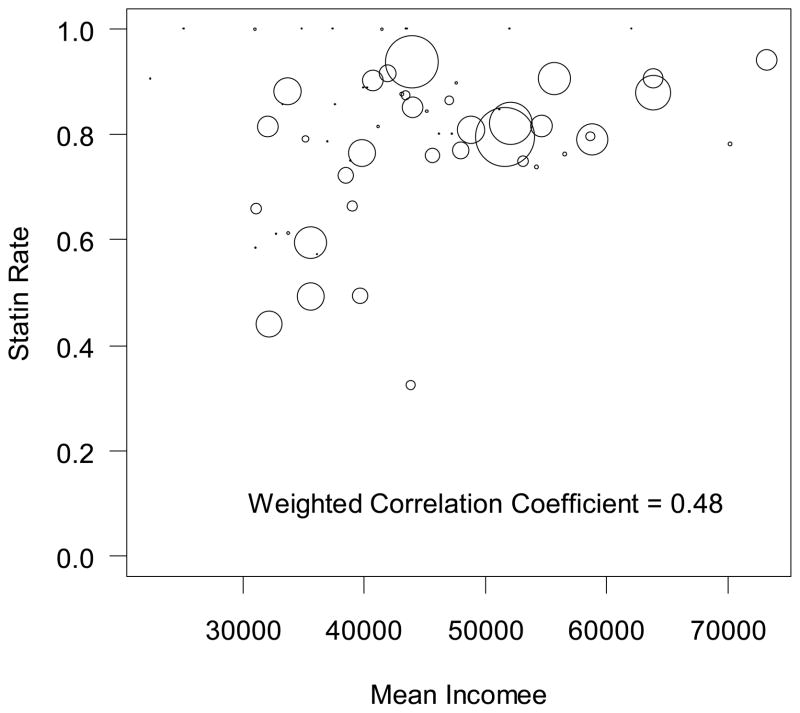
Treatment rates with statins decreased in a graded fashion going from higher to lower SES: 85.8% among quintile 5 to 72.5% for quintile 1 (Table 2). Compared with patients in the highest SES quintile, PAD patients in the lowest SES quintile were 16% less likely to be treated with statins (unadjusted rate ratio [RR], 0.84; 95% confidence interval [CI], 0.83–0.86, p<0.0001). Notably, sites with higher mean income among its patients had greater percentage of patients prescribed a statin medication (weighted correlation coefficient= 0.48) (Figure 1). After adjustment for the practice at which a patient received care, treatment differences by SES were markedly attenuated (adjusted RR for quintile 1 vs. 5, 0.97; 95% CI, 0.97–0.99, p=0.003). Further adjustment for clinical variables was associated with only a small attenuation of differences by SES (fully adjusted RR, 1.00; 95% CI, 0.99–1.01, p=0.772). A similar pattern was observed for quintiles 2, 3, and 4 (Table 3).
Table 2.
Medications Treatment Rates by Quintiles of Socioeconomic Status’*
| Median income by zip code | Total | P-Value | |||||
|---|---|---|---|---|---|---|---|
| Quintile 1 n = 12521 |
Quintile 2 n = 12513 |
Quintile 3 n = 12565 |
Quintile 4 n = 12511 |
Quintile 5 n = 12580 |
N=62,690 | ||
| Any antiplatelet: Clopidorgrel and/or Aspirin | 79.0 | 83.0 | 84.8 | 83.1 | 84.6 | 82.9 | < 0.001 |
| Statin | 72.5 | 77.6 | 83.2 | 81.5 | 85.8 | 80.1 | < 0.001 |
Continuous variables compared using linear trend test.
Categorical variables compared using Mantel-Haenszel trend test.
(Except as noted: KW = Kruskal-Wallis test)
Medications described among those eligible (without contraindication)
Figure 1. Correlation between a site’s mean patient income and rate of statin treatment.
The size of circles is a weighted representation of the number of patients with PAD at a practice.
Table 3.
Association of Socioeconomic Status with PAD Treatment
| Unadjusted | Adjustment for practice level variation | Further adjustment for clinical characteristics* | ||||
|---|---|---|---|---|---|---|
| RR, 95% CI | P-Value | RR, 95% CI | P-Value | RR, 95% CI | P-Value | |
| Statin Therapy | ||||||
| Quintile 5 | [Reference] | - | [Reference] | - | [Reference] | - |
| Quintile 4 | 0.95(0.94, 0.96) | <0.001 | 0.98(0.97, 1.00) | 0.0134 | 0.98(0.97, 1.00) | 0.007 |
| Quintile 3 | 0.97(0.96, 0.98) | <0.001 | 0.98(0.97, 1.00) | 0.0124 | 0.99(0.98, 1.00) | 0.172 |
| Quintile 2 | 0.90(0.89, 0.91) | <0.001 | 0.98(0.95, 1.00) | 0.0341 | 0.99(0.97, 1.01) | 0.327 |
| Quintile 1 | 0.84(0.83, 0.86) | <0.001 | 0.97(0.95, 0.99) | 0.0029 | 1.00(0.99, 1.01) | 0.772 |
| Any Antiplatelet ** | ||||||
| Quintile 5 | [Reference] | - | [Reference] | - | [Reference] | - |
| Quintile 4 | 0.99(0.98, 1.00) | 0.022 | 1.00(0.99, 1.01) | 0.556 | 1.01(1.00, 1.01) | 0.237 |
| Quintile 3 | 1.00(0.99, 1.01) | 0.946 | 0.99(0.98, 1.01) | 0.347 | 1.00(0.99, 1.01) | 0.788 |
| Quintile 2 | 0.98(0.97, 0.99) | <0.0001 | 1.00(0.99, 1.01) | 0.794 | 1.01(1.00, 1.02) | 0.071 |
| Quintile 1 | 0.93(0.91, 0.94) | <0.0001 | 0.98(0.97, 1.00) | 0.012 | 1.00(0.99, 1.01) | 0.878 |
Clinical characteristics adjusted model: model adjusted for practice, age, gender, history of myocardial infarction, revascularization in the past 12 months, insurance, congestive heart failure, diabetes, stroke, dyslipidemia, and tobacco use
Excluding patients on warfarin
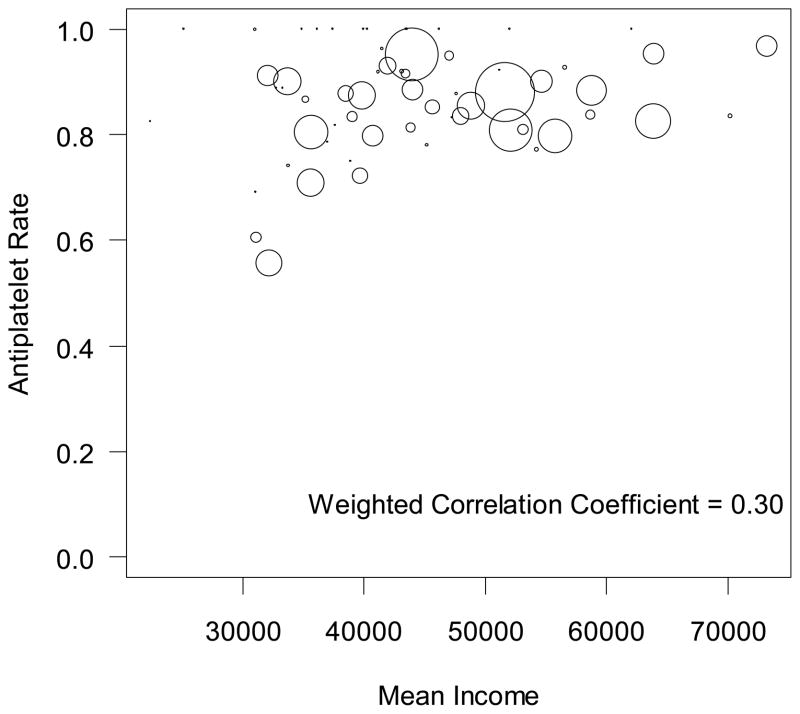
Rates of any antiplatelet were lowest among those in quintile 1 (79.0% among quintile 1 vs 84.6% among quintile 5) (see Table 2). Compared with patients in the highest SES quintile, patients in the lowest SES quintile were 7% less likely to be treated with any antiplatelet medication (unadjusted RR, 0.93; 95% CI, 0.91–0.94, p<0.0001). As with statin treatment, sites with higher median income among its patients had greater percentage of patients prescribed an antiplatelet agent (weighted correlation coefficient= 0.30) (Figure 2). After adjustment for the practice at which a patient received care, treatment differences by SES were nearly eliminated (adjusted RR, 0.98; 95% CI, 0.97–1.00, p=0.012). Further adjustment for the clinical characteristics of patients had minimal effect on attenuation of effect between the quintiles of SES (fully adjusted OR, 1.00; 95% CI, 0.99–1.01, p=0.878) (see Table 3).
Figure 2. Correlation between a site’s mean patient income and rate of antiplatelet treatment.
The size of circles is a weighted representation of the number of PAD patients at a practice.
Discussion
Among outpatients with PAD, we found that treatment with antiplatelet and statin therapies differed by SES. These differences, however, were largely explained by the clinical practice at which patients received care. Our findings suggest that initiatives to reduce disparities in medication treatment for PAD should target practices with high proportions of low SES patients.
Studies from different populations have demonstrated that cardiovascular risk factors and disease disproportionately affects those of lower SES,31–36 and among patients with cardiac disease, those with lower SES experience higher morbidity and mortality. 4,37–40 Patient level risk factors, such as increased health risk behaviors, may account for some of the increased morbidity and mortality seen in patients with low SES.4,41 However, identifying factors beyond patient risk factors and behaviors,36 which are not easily modifiable, are critical to quality initiatives that address the Institute of Medicine mandates to improve outcomes and reduce disparities. For instance, several studies have reported variation in adherence to evidence-based therapies for other cardiac conditions by SES, and may partly explain the association between lower SES and worse outcomes.37,42,43 However, these prior studies have not examined treatment differences by SES for PAD. Moreover, they have not examined the extent to which the site at which a patient receives his or her care influences treatment rates.
The present study expands on findings of other disparities research, and focuses on PAD, where outcomes research has been limited. Prior studies of disparities in PAD have focused on utilization rates of lower extremity revascularization by SES or reported rates of optimal medical therapy by insurance status5–9 This present study confirms findings similar to other cardiac disease states in that the use of secondary prevention treatments for PAD was lower among those of low SES. This finding highlights an important gap in the quality of care for patients with PAD, especially since the cost of aspirin and generic statins is low and should not pose significant barriers to patient access to these evidence-based therapies.
The present analysis further contributes to disparities research by highlighting the central role of the practice at which patients receive their care in explaining treatment differences by SES. We found that disparities in medication use between the highest and lowest SES were markedly attenuated after adjusting for site level variation; this suggests that differences in medication use were predominately explained by differences in sites that largely treat low SES compared with sites caring for largely higher SES patients. We believe these findings serve as an important paradigm for future efforts to reduce disparities in care, which will need to target clinical practices as intervention units and go beyond patient-level interventions. For PAD, future studies are needed to determine whether system-wide improvements at the practice level (e.g., identification of patients with PAD, initiation of secondary prevention medications, and physician education) or resource interventions at practices with high proportions of low SES patients (e.g., electronic medical systems, decision aids) will reduce disparities in treatment by SES. Quality improvement initiatives that provide feedback to sites by providing reports benchmarking performance of the site in relation to prespecified goals or national average for select performance metrics may help change behavior at a site.
There are several limitations to this study. First, we relied on patient diagnoses for PAD, which were self-reported by practices. It is possible that some patients were not classified as having PAD; however, we believe any misclassification would have been non-differential and are unlikely to have influenced our findings. Moreover, we were unable to examine severity of PAD, as we did not have physiologic (e.g., ankle-brachial index) or angiographic data on all patients. Similarly, we were unable to analyze the variability in use of medication by the specific PAD diagnosis (i.e. surgery vs non-invasive test, etc.) Second, we defined SES by median residential zip code income, which is a common strategy in previous studies,25–27 and did not examine other socioeconomic variables, such as educational level, which were not available in the PINNACLE registry. Third, our study was conducted among cardiology practices participating in PINNACLE, a quality improvement registry; therefore, treatment rates by SES may differ in non-participating practices including primary care centers. Given voluntary enrollment in this quality improvement initiative, it is possible that the rates of medication use are higher than expected in non-participating sites. We did not adjust for race in the clinical model given that it was frequently missing (nearly 50% of patients had missing data on this variable); however, most of the variation in treatment between the SES groups could be accounted for by practice-level variation. Furthermore, while it is possible that the differences in therapies by SES could be mediated by some, but not all, physicians within a practice, the current PINNACLE registry does not provide sufficient information on provider characteristics to allow us to currently examine this possibility. Regardless, further education and resources geared at practices with high proportions of low SES patients have the potential to improve adherence to therapies indicated for PAD. Finally, we were unable to examine longitudinal outcomes in this study.
Conclusion
Among patients with PAD, treatment with evidence-based antiplatelet and statin therapies differed by patients’ SES. These differences, however, were largely explained by the clinical practice at which patients received care, suggesting variation in treatment patterns across centers. Future efforts to reduce treatment disparities by SES in PAD and improve outcomes in these vulnerable populations should target practices serving high proportions of patients with low SES.
Acknowledgments
Funding
Dr. Subherwal was supported by a NIH T32 Training Grant (T32 HL 69749-9)
Dr. Smolderen was supported by the Outcomes Research post-doctoral fellowship awarded by the American Heart Association Pharmaceutical Roundtable and David and Stevie Spina [grant no. AHA: 0875149N], by the Netherlands Organization for Scientific Research [VENI grant no.: 916.11.179], and by an unrestricted grant from W.L. Gore & Associates, Inc. (Flagstaff, AZ).
Dr. Chan is supported by a Career Development Grant Award (K23HL102224) from the NHLBI.
The PINNACLE Registry is an initiative of the American College of Cardiology Foundation. Bristol-Myers Squibb and Pfizer Inc. are Founding Sponsors of the PINNACLE Registry.
Footnotes
- Manesh R. Patel received research grants from Johnson and Johnson, NHBLI, Astra Zeneca, Medtronic, and Maquet. Dr. Patel is a consultant/advisory board member for Bayer Healthcare, Genzyme, OrthoMcNeil Jansen, Pleuristem, Baxter
- Deepak Bhatt: receives research grants from Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, and The Medicines Company.
- All other authors have no disclosures or potential conflicts of interest to report.
Official NCDR Disclaimer
This research was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR). The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com.
References
- 1.America CoQoHCi, Medicine Io. Crossing the Quality Chasm: A New Health System for the 21st Century. The National Academies Press; 2001. [PubMed] [Google Scholar]
- 2.Rathore SS, Berger AK, Weinfurt KP, et al. Race, sex, poverty, and the medical treatment of acute myocardial infarction in the elderly. Circulation. 2000;102(6):642–648. doi: 10.1161/01.cir.102.6.642. [DOI] [PubMed] [Google Scholar]
- 3.Rathore SS, Foody JM, Wang Y, et al. Race, Quality of Care, and Outcomes of Elderly Patients Hospitalized With Heart Failure. JAMA: The Journal of the American Medical Association. 2003 May 21;289(19):2517–2524. doi: 10.1001/jama.289.19.2517. [DOI] [PubMed] [Google Scholar]
- 4.Alter DA, Chong A, Austin PC, et al. Socioeconomic Status and Mortality after Acute Myocardial Infarction. Annals of internal medicine. 2006 Jan 17;144(2):82–93. doi: 10.7326/0003-4819-144-2-200601170-00005. [DOI] [PubMed] [Google Scholar]
- 5.LaMorte WW, Scott TE, Menzoian JO. Racial differences in the incidence of femoral bypass and abdominal aortic aneurysmectomy in Massachusetts: relationship to cardiovascular risk factors. Journal of vascular surgery. 1995;21(3):422–431. doi: 10.1016/s0741-5214(95)70284-9. [DOI] [PubMed] [Google Scholar]
- 6.Huber TS, Wang JG, Wheeler KG, et al. Impact of race on the treatment for peripheral arterial occlusive disease. Journal of vascular surgery. 1999;30(3):417–426. doi: 10.1016/s0741-5214(99)70068-6. [DOI] [PubMed] [Google Scholar]
- 7.Eslami MH, Zayaruzny M, Fitzgerald GA. The adverse effects of race, insurance status, and low income on the rate of amputation in patients presenting with lower extremity ischemia. Journal of vascular surgery. 2007;45(1):55–59. doi: 10.1016/j.jvs.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 8.Meadows TA, Bhatt DL, Hirsch AT, et al. Ethnic differences in the prevalence and treatment of cardiovascular risk factors in US outpatients with peripheral arterial disease: Insights from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. American heart journal. 2009;158(6):1038–1045. doi: 10.1016/j.ahj.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Yeh D, Jones M, Schulman C, Karmacharya J, Velazquez OC. Uninsured South Florida vascular surgery patients are less likely to receive optimal medical management than their insured counterparts. Journal of vascular surgery. 2010;51(4):S4–S8. doi: 10.1016/j.jvs.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 10.Pande RL, Perlstein TS, Beckman JA, Creager MA. Secondary Prevention and Mortality in Peripheral Artery Disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011 doi: 10.1161/CIRCULATIONAHA.110.003954. CIRCULATIONAHA. 110.003954 v003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kröger K, Dragano N, Stang A, et al. An unequal social distribution of peripheral arterial disease and the possible explanations: results from a population-based study. Vascular Medicine. 2009 Nov 1;14(4):289–296. doi: 10.1177/1358863X09102294. [DOI] [PubMed] [Google Scholar]
- 12.Carson AP, Rose KM, Catellier DJ, et al. Cumulative socioeconomic status across the life course and subclinical atherosclerosis. Annals of epidemiology. 2007;17(4):296–303. doi: 10.1016/j.annepidem.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. The New England journal of medicine. 2000;342(3):145. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 14.A randomised, blinded trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) The Lancet. 1996;348(9038):1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 15.LINDHOLM LH. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Commentary. Lancet. 2003;361(9374):2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 16.Collaboration AT. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feringa HHH, van Waning VH, Bax JJ, et al. Cardioprotective medication is associated with improved survival in patients with peripheral arterial disease. Journal of the American College of Cardiology. 2006;47(6):1182–1187. doi: 10.1016/j.jacc.2005.09.074. [DOI] [PubMed] [Google Scholar]
- 18.Leng GC, Lee AJ, FOWKERS FGR, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. International journal of epidemiology. 1996;25(6):1172. doi: 10.1093/ije/25.6.1172. [DOI] [PubMed] [Google Scholar]
- 19.Steg PG, Bhatt DL, Wilson PWF, et al. One-Year Cardiovascular Event Rates in Outpatients With Atherothrombosis. JAMA: The Journal of the American Medical Association. 2007 Mar 21;297(11):1197–1206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 20.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of High and Low Ankle Brachial Index to All-Cause and Cardiovascular Disease Mortality. Circulation. 2004 Feb 17;109(6):733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 21.Criqui MH, Langer RD, Fronek A, et al. Mortality over a Period of 10 Years in Patients with Peripheral Arterial Disease. New England Journal of Medicine. 1992;326(6):381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 22.Criqui MH, Ninomiya JK, Wingard DL, Ji M, Fronek A. Progression of Peripheral Arterial Disease Predicts Cardiovascular Disease Morbidity and Mortality. Journal of the American College of Cardiology. 2008;52(21):1736–1742. doi: 10.1016/j.jacc.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan PS, Oetgen WJ, Buchanan D, et al. Cardiac Performance Measure Compliance in Outpatients: The American College of Cardiology and National Cardiovascular Data Registry’s PINNACLE (Practice Innovation And Clinical Excellence) Program. J Am Coll Cardiol. 2010 Jun 29;56(1):8–14. doi: 10.1016/j.jacc.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan PS, Oetgen WJ, Spertus JA. The Improving Continuous Cardiac Care (IC3) Program and Outpatient Quality Improvement. The American Journal of Medicine. 2010;123(3):217–219. doi: 10.1016/j.amjmed.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Burstin HR, Lipsitz SR, Brennan TA. Socioeconomic status and risk for substandard medical care. JAMA: The Journal of the American Medical Association. 1992;268(17):2383. [PubMed] [Google Scholar]
- 26.Santry HP, Gillen DL, Lauderdale DS. Trends in Bariatric Surgical Procedures. JAMA: The Journal of the American Medical Association. 2005 Oct 19;294(15):1909–1917. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 27.Rao SV, Schulman KA, Curtis LH, Gersh BJ, Jollis JG. Socioeconomic status and outcome following acute myocardial infarction in elderly patients. Archives of internal medicine. 2004;164(10):1128. doi: 10.1001/archinte.164.10.1128. [DOI] [PubMed] [Google Scholar]
- 28.Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (Updating the 2005 Guideline) Circulation. 2011 Sep 29;2011 [Google Scholar]
- 29.Olin JW, Allie DE, Belkin M, et al. ACCF/AHA/ACR/SCAI/SIR/SVM/SVN/SVS 2010 Performance Measures for Adults With Peripheral Artery Disease: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures, the American College of Radiology, the Society for Cardiac Angiography and Interventions, the Society for Interventional Radiology, the Society for Vascular Medicine, the Society for Vascular Nursing, and the Society for Vascular Surgery (Writing Committee to Develop Clinical Performance Measures for Peripheral Artery Disease) Developed in Collaboration With the American Association of Cardiovascular and Pulmonary Rehabilitation; the American Diabetes Association; the Society for Atherosclerosis Imaging and Prevention; the Society for Cardiovascular Magnetic Resonance; the Society of Cardiovascular Computed Tomography; and the PAD Coalition Endorsed by the American Academy of Podiatric Practice Management. J Am Coll Cardiol. 2010 Dec 14;56(25):2147–2181. doi: 10.1016/j.jacc.2010.08.606. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein H. Multilevel statistical models. Vol. 847. Wiley; 2010. [Google Scholar]
- 31.Cooper R, Cutler J, Desvigne-Nickens P, et al. Trends and Disparities in Coronary Heart Disease, Stroke, and Other Cardiovascular Diseases in the United States: Findings of the National Conference on Cardiovascular Disease Prevention. Circulation. 2000 Dec 19;102(25):3137–3147. doi: 10.1161/01.cir.102.25.3137. [DOI] [PubMed] [Google Scholar]
- 32.Smith SC, Clark LT, Cooper RS, et al. Discovering the Full Spectrum of Cardiovascular Disease. Circulation. 2005 Mar 15;111(10):e134–e139. doi: 10.1161/01.CIR.0000157743.54710.04. [DOI] [PubMed] [Google Scholar]
- 33.Lynch J, Kaplan GA, Salonen R, Salonen JT. Socioeconomic Status and Progression of Carotid Atherosclerosis: Prospective Evidence From the Kuopio Ischemic Heart Disease Risk Factor Study. Arteriosclerosis, thrombosis, and vascular biology. 1997 Mar 1;17(3):513–519. doi: 10.1161/01.atv.17.3.513. [DOI] [PubMed] [Google Scholar]
- 34.Deans KA, Bezlyak V, Ford I, et al. Differences in atherosclerosis according to area level socioeconomic deprivation: cross sectional, population based study. BMJ. 2009 Oct 27;2009:339. doi: 10.1136/bmj.b4170. 00:00:00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winkleby MA, Robinson TN, Sundquist J, Kraemer HC. Ethnic Variation in Cardiovascular Disease Risk Factors Among Children and Young Adults. JAMA: The Journal of the American Medical Association. 1999 Mar 17;281(11):1006–1013. doi: 10.1001/jama.281.11.1006. [DOI] [PubMed] [Google Scholar]
- 36.Albert MA, Glynn RJ, Buring J, Ridker PM. Impact of Traditional and Novel Risk Factors on the Relationship Between Socioeconomic Status and Incident Cardiovascular Events. Circulation. 2006 Dec 12;114(24):2619–2626. doi: 10.1161/CIRCULATIONAHA.106.660043. [DOI] [PubMed] [Google Scholar]
- 37.Kapral MK, Wang H, Mamdani M, Tu JV. Effect of Socioeconomic Status on Treatment and Mortality After Stroke. Stroke. 2002 Jan 1;33(1):268–275. doi: 10.1161/hs0102.101169. [DOI] [PubMed] [Google Scholar]
- 38.Cox AM, McKevitt C, Rudd AG, Wolfe CDA. Socioeconomic status and stroke. The Lancet Neurology. 2006;5(2):181–188. doi: 10.1016/S1474-4422(06)70351-9. [DOI] [PubMed] [Google Scholar]
- 39.Rathore SS, Masoudi FA, Wang Y, et al. Socioeconomic status, treatment, and outcomes among elderly patients hospitalized with heart failure: findings from the National Heart Failure Project. American heart journal. 2006;152(2):371–378. doi: 10.1016/j.ahj.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koch CG, Li L, Kaplan GA, et al. Socioeconomic Position, Not Race, Is Linked to Death After Cardiac Surgery. Circulation: Cardiovascular Quality and Outcomes. 2010 May 1;3(3):267–276. doi: 10.1161/CIRCOUTCOMES.109.880377. [DOI] [PubMed] [Google Scholar]
- 41.Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality. JAMA: The Journal of the American Medical Association. 1998;279(21):1703. doi: 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- 42.Philbin EF, McCullough PA, DiSalvo TG, Dec GW, Jenkins PL, Weaver WD. Socioeconomic Status Is an Important Determinant of the Use of Invasive Procedures After Acute Myocardial Infarction in New York State. Circulation. 2000 Nov 7;102(suppl 3):III-107–III-115. doi: 10.1161/01.cir.102.suppl_3.iii-107. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez AF, Fonarow GC, Liang L, et al. Sex and Racial Differences in the Use of Implantable Cardioverter-Defibrillators Among Patients Hospitalized With Heart Failure. JAMA: The Journal of the American Medical Association. 2007 Oct 3;298(13):1525–1532. doi: 10.1001/jama.298.13.1525. [DOI] [PubMed] [Google Scholar]