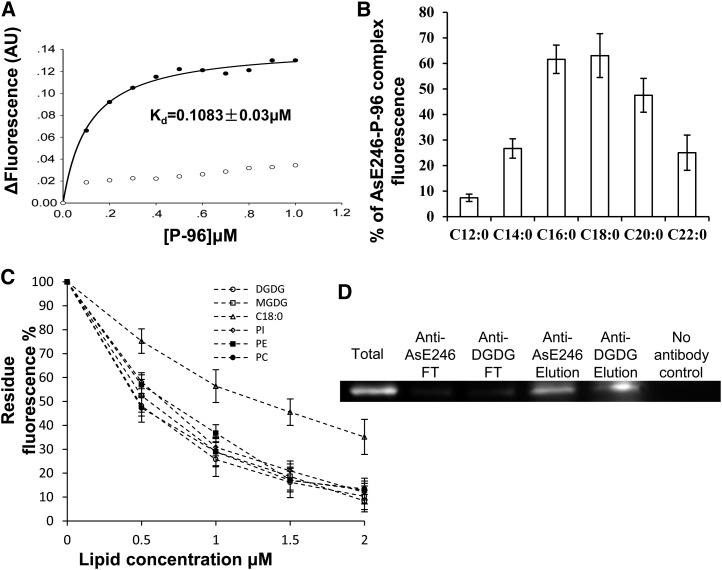
Figure 1.
Lipid binding activity assay of AsE246. A, Association of P-96 with ΔAsE246. Purified ΔAsE246 (final concentration, 0.5 μm) was incubated in 10 mm MOPS, pH 7.2. Increasing amounts of P-96 (from a 30 μm solution in ethanol) were added, and the fluorescence intensity was measured at 378 nm (excitation at 343 nm). ΔAsE246, incubated with increasing concentrations of P-96, exhibited saturation binding defined by Kd = 0.1083 ± 0.03 μm (circles). No saturation binding was observed (squares) when P-96 was incubated with GST in the control experiment. B, Binding competition between P-96 and various unlabeled fatty acids. Purified ΔAsE246 (final concentration 0.5 μm) was incubated in 10 mm MOPS, pH 7.2, with 1 μm P-96 (from a 30 μm solution in ethanol). Various unlabeled fatty acids were added from concentrated solutions in ethanol (final concentration, 10 μm). Fluorescence intensity was measured at 378 nm (excitation at 343 nm) after its stabilization. C, Binding ability competition between P-96 and natural lipid, PC, PE, PI, DGDG, and its precursor MGDG. Purified ΔAsE246 (final concentration, 0.5 μm) was incubated in 10 mm MOPS, pH 7.2, with 1 μm P-96 (from a 30 μm solution in ethanol). Increasing amounts of unlabeled lipids were added from concentrated solutions. D, Co-IP of AsE246 with DGDG. The wild-type Chinese milk vetch nodules inoculated with M. huakuii extracts were incubated first with anti-DGDG antibody and then with immobilized Protein G beads. Proteins bound to the beads were separated on SDS-PAGE and immunoblotted with anti-AsE246 antibody.