A noninvasive system enables quantification of metal uptake and storage in micoralgae.
Abstract
We have developed a fluorescence resonance energy transfer (FRET)-based heavy metal biosensor for the quantification of bioavailable free heavy metals in the cytoplasm of the microalga Chlamydomonas reinhardtii. The biosensor is composed of an end-to-end fusion of cyan fluorescent protein (CFP), chicken metallothionein II (MT-II), and yellow fluorescent protein (YFP). In vitro measurements of YFP/CFP fluorescence emission ratios indicated that the addition of metals to the purified biosensor enhanced FRET between CFP and YFP, consistent with heavy metal-induced folding of MT-II. A maximum YFP/CFP FRET ratio of 2.8 was observed in the presence of saturating concentrations of heavy metals. The sensitivity of the biosensor was greatest for Hg2+ followed by Cd2+ ≈ Pb2+ > Zn2+ > Cu2+. The heavy metal biosensor was unresponsive to metals that do not bind to MT-II (Na+ and Mg2+). When expressed in C. reinhardtii, we observed a differential metal-dependent response to saturating external concentrations (1.6 mm) of heavy metals (Pb2+ > Cd2+) that was unlike that observed for the isolated biosensor (in vitro). Significantly, analysis of metal uptake kinetics indicated that equilibration of the cytoplasm with externally applied heavy metals occurred within seconds. Our results also indicated that algae have substantial buffering capacity for free heavy metals in their cytosol, even at high external metal concentrations.
Many proteins utilize metals to stabilize their structures or as cofactors to catalyze redox and other chemical reactions. Metals such as zinc, copper, iron, magnesium, cobalt, and manganese are required by most living organisms for their normal cellular functions. Essential metals are often present at low concentrations in the environment, however, and must be imported into cells, often at the expense of energy (Hanikenne et al., 2005; Merchant et al., 2006). In contrast to essential metals, toxic metals such as cadmium, lead, and mercury can disrupt cellular functions by competing with essential metals for their metal-binding sites and/or by altering the redox state of cells. Exposure of organisms to high concentrations of toxic metals can impair their cellular functions, growth, and reproduction. To prevent metal-induced cellular anomalies, organisms have evolved a variety of strategies to reduce the toxicity of heavy metals. One such strategy involves the selective binding of toxic metals in the cytoplasm by metal-binding proteins and other small molecules. As discussed below, both enzymatically and ribosomally synthesized Cys-rich peptides, including phytochelatins and metallothioneins (MTs), are utilized by a variety of organisms to sequester toxic heavy metals, including cadmium, mercury, lead, silver, and gold. The peptides may also serve as storage reserves for essential metals such as copper and zinc (Cobbett and Goldsbrough, 2002).
Phytochelatins are enzymatically synthesized polypeptides containing repeating units of (γ-Glu-Cys)n-Gly, where n = 2 to 11 (Rauser, 1990), whereas MTs are genetically encoded, ribosomally synthesized polypeptides (Cobbett and Goldsbrough, 2002). MTs have molecular mass values ranging from 6 to 7 kD and contain approximately 20 conserved Cys residues (Cobbett and Goldsbrough, 2002; Romero-Isart and Vasák, 2002). Metals are characteristically bound to MT via the thiolate sulfur ligands of Cys residues (Kägi, 1991). It is estimated that the metal-saturated MT contains about 10% thiolate sulfur and bound metals by mass (Romero-Isart and Vasák, 2002). Structural analyses of metal-free and metal-complexed MTs demonstrated that MTs undergo a structural transition from a metal-free random-coil structure to a metal-bound compact dumbbell-shaped structure having metal saturated α- and β-domains (Pearce et al., 2000; Romero-Isart and Vasak, 2002; Hong and Maret, 2003). The N-terminal β-domain binds three metal ion equivalents, and the C-terminal α-domain binds four metal ion equivalents (Romero-Isart and Vasák, 2002; Vasák, 2005). Furthermore, several decades of work on MTs have provided a great deal of information regarding their metal-binding affinity, specificity, and domain selectivity for select metals (Cobbett and Goldsbrough, 2002; Romero-Isart and Vasák, 2002; Vasák, 2005).
Fluorescence resonance energy transfer (FRET) involves the nonradioactive transfer of energy between the excited state of a luminescent or fluorescent donor molecule and a nearby acceptor molecule that has overlapping excited state transitions. Proteins that are modified to have efficient energy donor and acceptor domains and that undergo structural changes upon binding a specific ligand are good candidates for FRET-based sensors. For ligand-specific FRET-based biosensors, the distance and/or the orientation between the energy donor and acceptor molecules is changed upon ligand binding in a concentration-dependent manner (Selvin, 1995; Weiss, 2000; Hong and Maret, 2003; Looger et al., 2005). Relevant to this discussion, a FRET-based biosensor with GFP variants fused to MT was previously shown to be an effective means to monitor metal release during nitric oxide-induced signaling in endothelial cells (Pearce et al., 2000).
Unicellular algae such as Chlamydomonas species are often found in areas that might be contaminated by toxic heavy metals (Merchant et al., 2006). Chlamydomonas species have also been shown to sequester toxic metals (e.g. cadmium and mercury) and have potential use for bioremediation of these metals (Cai et al., 1999; Adhiya et al., 2002; Siripornadulsil et al., 2002; He et al., 2011; Priyadarshani et al., 2011). To determine the kinetics and selectivity of exogenous heavy metal uptake as well as free heavy metal concentration in the cytoplasm of Chlamydomonas species, we developed an MT, FRET-based metal-binding sensor and expressed this in the cytoplasm of the unicellular green alga Chlamydomonas reinhardtii. We demonstrate that heavy metal uptake is rapid in C. reinhardtii and that cytoplasmic free heavy metal concentrations are substantially lower than exogenous free heavy metal concentrations, implying that heavy metals are rapidly sequestered by various biological molecules in the cell.
RESULTS
In Vitro Metal Biosensor
A FRET-based heavy metal biosensor was successfully constructed by expressing a chicken metallothionein II (MT-II) protein fused between cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) variants of GFP (CFP, maximum excitation/emission wavelength [λex] 440 nm/λem 485 nm; YFP, λex 515 nm/λem 527 nm). To prevent FRET artifacts associated with the potential dimerization of CFP or YFP (dissociation constant [Kd] of approximately 100 µm), we introduced the L221K mutation into both CFP and YFP to reduce the likelihood of fluorophore dimerization and intermolecular FRET (Tsien, 1998; Zacharias et al., 2002). The resulting fusion protein consists of an N-terminal CFP linked to MT-II and a C-terminal YFP (CMY) having a combined mass of 61 kD (Supplemental Fig. S1).
To characterize the FRET response of CMY to metals, we overexpressed CMY in Escherichia coli as a maltose-binding protein (MBP)-CMY fusion protein. The purified MBP-CMY fusion protein was then digested with factor Xa, and the two proteins were separated from each other by amylose affinity separation of MBP from CMY.
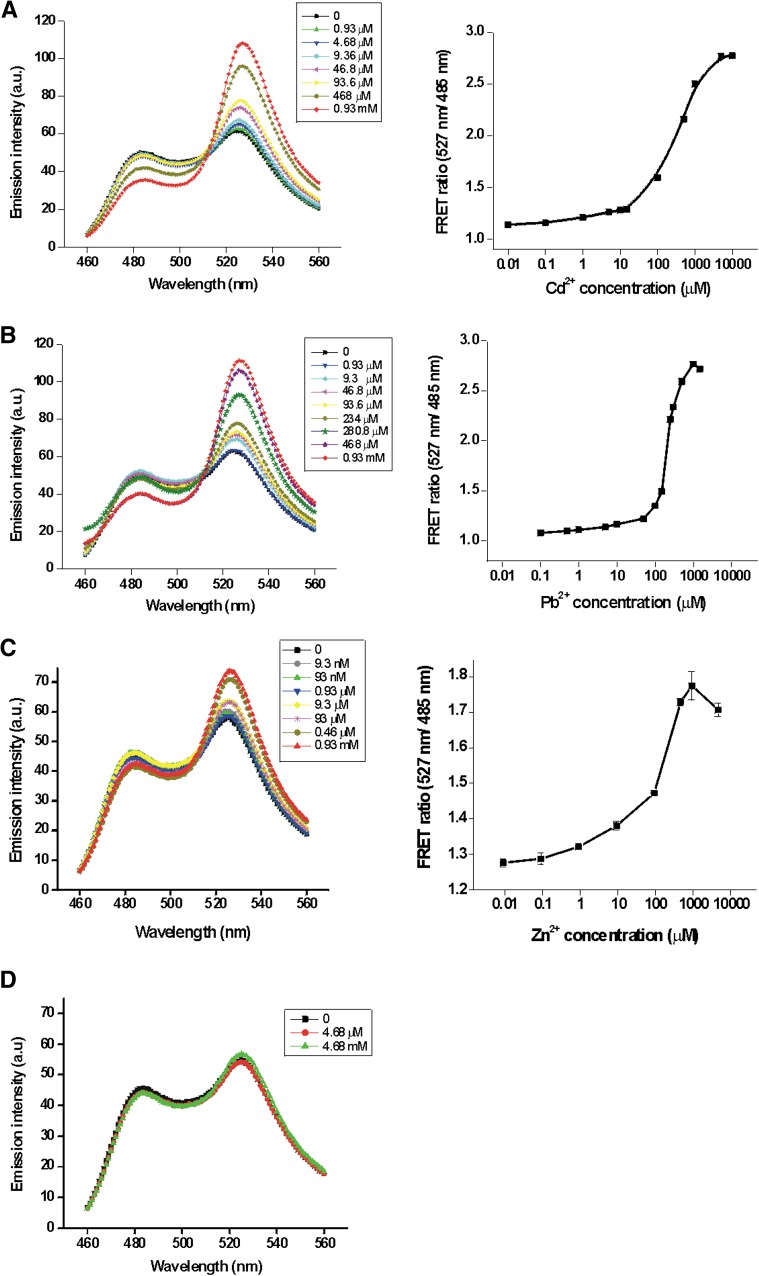
MTs are rich in Cys residues, which when oxidized could lead to disulfide bond formation. The formation of intermolecular and intramolecular disulfide bonds could result in lowered metal sensitivity and altered FRET efficiencies. Earlier studies have shown that treatment of MT with 2-mercaptoethanol reduced oxidized thiol groups without affecting the protein affinity toward metals (Suzuki and Maitani, 1981). Therefore, for our in vitro assays, CMY was first reduced with 2-mercaptoethanol (15 mm) followed by the removal of 2-mercaptoethanol prior to metal-binding assays. In the absence of metals that bind to MT-II, the CMY biosensor had a YFP/CFP fluorescence emission ratio of about 1.2. But in the presence of metals such as Cd2+, Pb2+, and Zn2+ that bind to MT-II, there was a concentration-dependent increase in FRET (Fig. 1). FRET analyses indicated that the half-maximal metal saturation concentrations for Cd2+, Pb2+, and Zn2+ were approximately 225, 205, and 310 µm, respectively. The maximum YFP/CFP fluorescence ratio for Cd2+ and Pb2+ was 2.8 (Fig. 1, A and B). In contrast, the maximum YFP/CFP fluorescence ratio was only 1.8 for saturating concentrations of Zn2+, suggesting an enhanced binding structural interaction for Cd2+ and Pb2+ (Fig. 1C). This apparent enhanced metal selectivity of Cd2+ and Pb2+ was similar to the previously observed metal selectively of MT-II (Kägi, 1991), with the exception that the sensitivity to mercury binding induced a lower FRET response than was expected, possibly reflecting its potential interactions with YFP and CFP (Fig. 2B). To determine if CMY underwent similar conformational changes when exposed to metals that are not known to bind to MT-II, we measured the CMY FRET response following exposure to Na+ and Mg2+, metals that do not bind specifically to MT-II. As expected, the CMY sensor exhibited no FRET response to Mg2+ exposure (Fig. 1D) and Na+ (data not shown), consistent with the lack of known interaction between MT-II and these metals.
Figure 1.
Metal-induced FRET ratio changes. Concentration-dependent increases in FRET ratio (527 nm/485 nm) were observed with various concentrations of Cd2+ (A), Pb2+ (B), Zn2+ (C), and Mg2+ (D). FRET measurements with different protein preparations had identical FRET changes for the metals indicated. Measurements were made in triplicate, and sd values are represented as error bars. a.u., Arbitrary units. [See online article for color version of this figure.]
Figure 2.
CMY FRET response to copper and mercury is impaired at high metal concentrations. Cu2+ (A) and Hg2+ (B) were titrated with CMY to monitor FRET changes. Measurement parameters were as follows: λex 440 nm/slit 5 nm, λem 460 to 560 nm/slit 5 nm. [See online article for color version of this figure.]
It is noted that the purified and intact MBP-CMY fusion protein exhibited only limited FRET changes, even at saturating concentrations of metals (1 mm or greater Pb2+ or Cd2+) known to bind to MT-II. The YFP/CFP fluorescence emission ratio for MBP-CMY in the presence of saturating metal concentrations (1 mm or greater) was only 0.5 nM greater than in the presence of nonsaturating (5 nm) metal concentrations (data not shown). This difference was approximately 3 times less than for the isolated CMY protein without MBP. We attribute the reduced metal-binding-induced FRET response to reduced conformational changes induced by the bulky MBP (42 kD) attached to the CMY sensor.
CMY Responses to Cu2+ and Hg2+
Earlier work with an MT-FRET metal biosensor expressed in Chinese hamster ovary crude cell lysates demonstrated a Cu+-induced increase in FRET (Pearce et al., 2000). However, our attempts to elicit FRET changes in vitro with Cu2+ (concentration greater than 90 nm) did not result in any appreciable increase in the YFP/CFP ratio; rather, it diminished CFP and YFP fluorophore intensities (Fig. 2A). This fluorescence loss may be attributed to Cu2+-dependent fluorescence quenching of CFP and YFP fluorophores (Zimmer, 2002). In contrast to copper, the YFP/CFP fluorescence ratio increased with increasing concentrations of Hg2+ (up to approximately 9 µm). However, pronounced fluorescence quenching was observed with high mercury concentrations (10 µm or greater; Fig. 2B). These results suggest that, in addition to inducing FRET-based shifts in fluorescence emission, Cu2+ and Hg2+ may affect the fluorophores directly, altering the sensitivity of the biosensor (Tsien, 1998; Nagai et al., 2002; Zimmer, 2002).
Algal FRET Biosensor
It is well established that algae sequester large quantities of metals to their cell walls (Adhiya et al., 2002; Mehta and Gaur, 2005; Tüzün et al., 2005). To reduce the effects of cell wall-mediated metal binding, we used a cell wall-less strain of C. reinhardtii (CC-425) expressing the CMY biosensor to monitor heavy metal uptake. Transgenic algae carrying the CMY gene construct were developed for the constitutive expression of CMY under the control of the β-tubulin promoter (Siripornadulsil et al., 2002). Identification of positive integration of the CMY transgene into the C. reinhardtii nuclear DNA was confirmed by PCR analysis using genomic DNA preparations. The expression of the CMY biosensor was further verified by fluorescence microscopy (Supplemental Fig. S2).
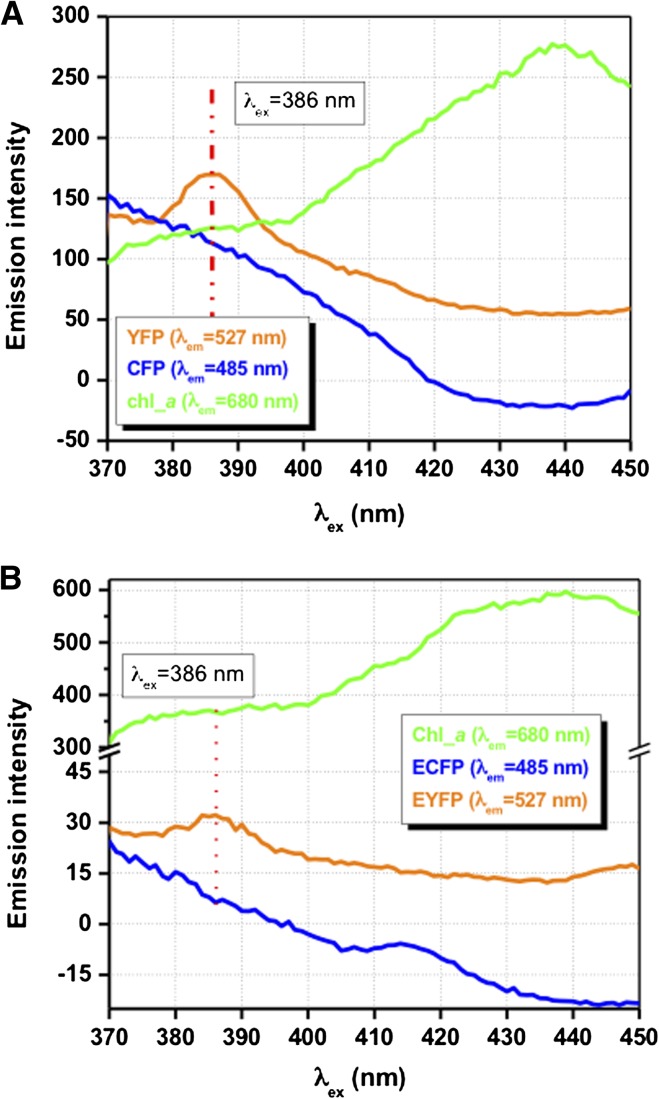
The CFP fluorophore used in the CMY FRET construct has a λex between 430 and 440 nm. Chlorophyll a, a light-harvesting pigment in C. reinhardtii, also absorbs strongly at 440 nm, however. To investigate possible quenching of CMY excitation and fluorescence by chlorophyll a, spectrofluorometric measurements were carried out with the wild type (CC-2137) and five independent transgenic algae expressing CMY over a range of excitation wavelengths. Excitation scans (λex 370–450 nm/slit 10 nm) at fixed emission wavelengths for CFP (λem 485 nm/slit 5 nm), YFP (λem 527 nm/slit 5 nm), and chlorophyll a (λem 680 nm/slit 5 nm) were recorded. Unexpectedly, the fluorescence emission spectra of wild-type cells (Fig. 3B) indicated the presence of unknown fluorescence emission peaks having emission wavelengths similar to CFP and YFP. However, this fluorescence background was experimentally determined to be an artifact associated with the specific trace metal solution used in the algae growth medium. This trace metal-induced artifact was presumably due to the formation of organometallic adducts during heat sterilization of the medium. Importantly, the yellow and blue background fluorescence emission peak intensities were not affected by the addition of the range of metals being tested in this study. In order to eliminate the minor fluorescence contributions from media and algal cells, the background fluorescence yield from wild-type culture was subtracted from each experimental measurement made with CMY-expressing cells. Significantly, transgenic algae expressing CMY exhibited reduced chlorophyll a fluorescence per cell relative to wild-type cells for a range of excitation wavelengths tested (Fig. 3, compare A and B). These results suggest that the CFP fluorophore competed with chlorophyll for blue light absorption. To address this concern, we determined empirically that the optimal excitation wavelength range for maximal CFP and YFP fluorescence emission yields and reduced chlorophyll absorption in vivo was between 380 and 390 nm (Fig. 3A).
Figure 3.
Fluorescence emission spectra of CMY-expressing and wild-type cells as a function of excitation wavelength. Fluorescence yields were measured at several wavelengths including: λem = 485 nm, corresponding to the maximum fluorescence wavelength for CFP, at λem = 527 nm, corresponding to the maximum fluorescence wavelength for YFP, and at λem = 680 nm, corresponding to the maximum fluorescence wavelength for chlorophyll. CMY-expressing C. reinhardtii cells (GBCMY33; A) and wild-type cells having identical cell numbers (4.0 × 105 cells mL−1; B) were assayed. Excitation wavelengths ranged between λex 370 and 450 nm (slit 10 nm). Note the difference in the fluorescence intensity scale between A and B. [See online article for color version of this figure.]
Toxic Heavy Metal Detection Using the Algal CMY Biosensor
To determine the free concentration of bioavailable MT-II-binding metals in algal cells, we measured CMY FRET changes as a function of different external metal concentrations. The commonly used C. reinhardtii growth medium Tris-acetate-phosphate (TAP) has high concentrations of phosphate, a potential chelator of metals. In silico metal speciation analysis of TAP medium using MINEQL+ and metal salt solutions indicated the potential for heavy metal complex formation with both the organic and inorganic components of the medium. Significantly, free heavy metal concentrations were reduced by as much as 3 orders of magnitude from the applied concentrations in TAP medium due to metal speciation (Supplemental Table S1). Modeling studies predicted that Cd3(PO4)2 and Pb5(PO4)3Cl (pyromorphite) were the prominent complexed metal species in TAP medium. To reduce metal complex formation by inorganic phosphate, we substituted glycerophosphate for free phosphate in the TAP medium (Collard and Matagne, 1990).
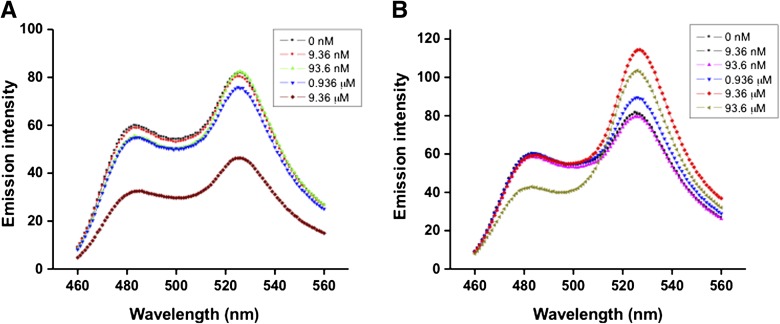
Five independent transgenic cell lines expressing CMY were used for metal studies (Supplemental Fig. S2). Upon the addition of heavy metals, each of these cell lines showed identical, metal-specific FRET responses (data not shown). One of the transgenic cell lines (GBCMY 5) exhibiting the highest CMY fluorescence response to added heavy metals was chosen for further studies. Algal FRET measurements were initiated immediately after metal addition to cells (4 × 105 cells mL−1). Overlays of the λem spectra of algal responses to different concentrations of metals demonstrated a metal concentration-dependent change in FRET (Fig. 4).
Figure 4.
In vivo fluorescence responses of the CMY biosensor to various metals. Fluorescence emission spectra (λex 386 nm/slit 10 nm, λem 460–560 nm/slit 5 nm) are shown following the addition of MT-II-binding metals (Cd2+ [A], Pb2+ [B], and Cu2+ [C]) and non-MT-II-binding metals (Na+ [D] and Mg2+ [E]) to transgenic algae. Background fluorescence detected in wild-type algae was subtracted from the fluorescence spectra of transgenic algae. The plotted metal concentrations are the external concentrations applied to the cells. a.u., Arbitrary units. [See online article for color version of this figure.]
The fluorescence emissions from transgenic cells were normalized by subtracting the background-nonspecific fluorescence for each metal treatment and are listed in Table I. The background fluorescence emission yield for both wild-type CC-2137 and the cell wall-less strain (CC425) were determined to be about 15% of that of transgenic cells (Supplemental Fig. S3) and remained unaltered with metal addition. Interestingly, the initial CMY ratio (0.86) of cells unexposed to metals that bind to MT-II was less than that observed for isolated CMY expressed in E. coli and chemically reduced in vitro with 2-mercaptoethanol (Fig. 1). These results suggest that the sulfhydryls involved in metal binding in CMY were fully reduced in the cytosol of transgenic algal cells. As shown in Figure 4 and Table I, incubation of cells with Cd2+ and Pb2+ but not with Na+ and Mg2+ induced detectable FRET responses in CMY-expressing cells. At the highest metal concentrations tested, Pb2+ induced the largest FRET change of 0.76 (Fig. 4B; Table I), followed by Cd2+ (0.25) and Cu2+ (0.15). Compared with the in vitro CMY FRET responses, the algal cytosolic FRET responses for similar externally applied heavy metal concentrations of Pb2+ and Cd2+ were 6- to 25-fold lower than those observed for the isolated protein, reflecting reduced free metal concentrations in vivo.
Table I. Algae biosensor and metal-specific FRET ratio responses.
Normalized transgenic algae FRET ratio (527 nm/485 nm) responses for no metal addition and highest metal concentration tested (1.57 mm) and CMY FRET ratio differences are shown. NA, Not applicable; ND, not determined.
| Metal Ion | In Vivo YFP/CFP FRET Efficiency Ratioa | YFP/CFP FRET Ratio with 1.6 mm External Metal Addition | Change in YFP/CFP FRET Ratio | Estimated Internal Metal Concentration (Percentage of External Concentration) |
|---|---|---|---|---|
| Cd2+ | 0.90 | 1.15 | 0.25 | 60–70 µm (4.0%) |
| Pb2+ | 0.90 | 1.64 | 0.74 | 240–260 µm (16%) |
| Cu2+ | 0.90 | 1.05 | 0.15 | ND |
| Mg2+ | 0.94 | 0.88 | −0.06 | NA |
| Na+ | 0.88 | 0.85 | −0.03 | NA |
In contrast to monovalent copper, which binds to MT and would induce a CMY FRET response (Pearce et al., 2000), divalent copper does not induce a CMY FRET response but did cause fluorescence quenching at high metal concentrations (Fig. 2A), We observed only a small CMY FRET response (0.15; Fig. 4C) at the highest Cu2+ concentrations tested (Table I). Interestingly, the in vivo FRET response was accompanied by no apparent fluorescence quenching, as observed in vitro (compare Figs. 2A and 4C). This disparity may reflect the poor uptake of Cu2+ by C. reinhardtii cells. Copper uptake in C. reinhardtii is highly regulated, involving a cupric reductase and high-affinity copper transporter, which results in the delivery of reduced monovalent copper (Hill et al., 1996). We speculate that the small FRET ratio change (527 nm/485 nm) observed in the presence of copper could be caused by CMY binding to reduced Cu+ delivered by this copper uptake system.
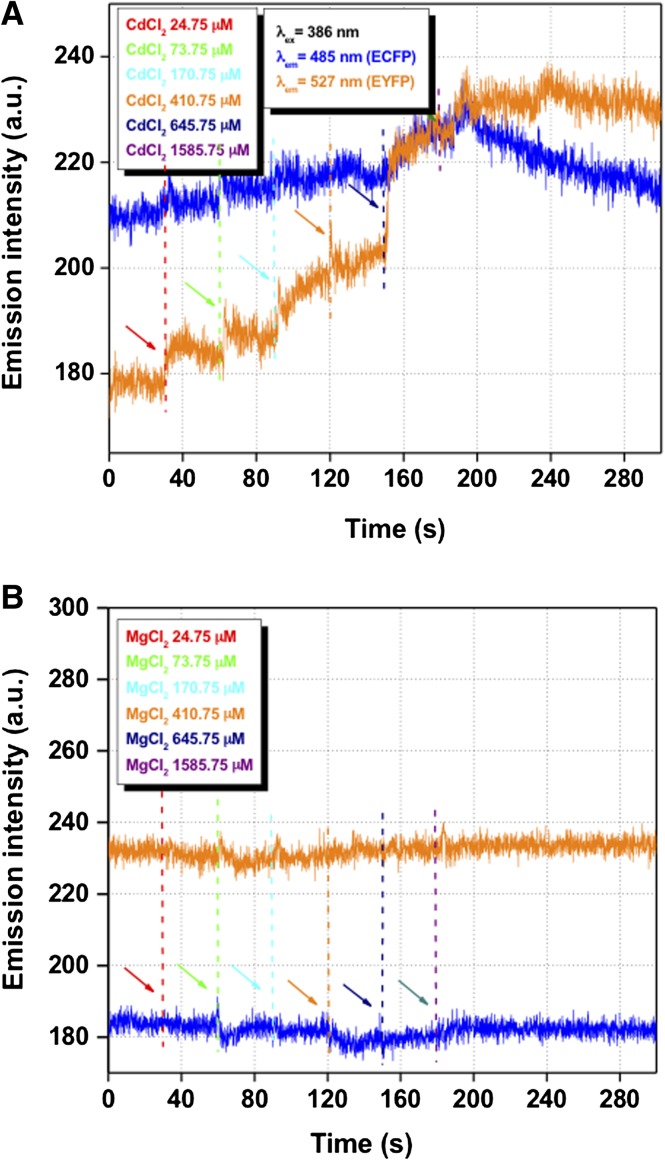
To verify the metal selectivity of the in vivo CMY FRET responses, we exposed the CMY-expressing algae to Na+ and Mg2+ and measured their CMY FRET response. The addition of these metal salts showed no observable change in the YFP/CFP fluorescence ratio (Fig. 4; Table I), demonstrating the metal selectivity of the CMY FRET response. We also determined the rate of metal uptake by algal cells by temporally monitoring FRET changes following the addition of heavy metals. Based on the kinetics of the CMY FRET response, we estimate that saturable cadmium uptake was complete within milliseconds of metal addition to the cell cultures (Fig. 5).
Figure 5.
Kinetics of CFP (blue) and YFP (yellow) fluorescence responses to added toxic metals in transgenic algae. Arrows indicate the addition of metals at the concentrations noted. CFP (λex 386 nm/slit 10 nm, λem 485 nm/slit 5 nm) and YFP (λex 386 nm/slit 10 nm, λem 527 nm/slit 5 nm) fluorescence was measured after the addition of Cd2+ (A) or Mg2+ (B). a.u, Arbitrary units. [See online article for color version of this figure.]
Finally, based on the FRET responses, it was determined that the magnitude of the metal-specific CMY FRET response in algae was Pb2+ > Cd2+ >> Cu2+. This order of response sensitivity is different for MT metal selectivity (Cu+ > Cd2+ > Pb2+) in vitro (Romero-Isart and Vasák, 2002; Priyadarshani et al., 2011), suggesting that there was a differential free cytoplasmic metal concentration for each metal tested, even if the external metal concentrations were identical. Heavy metals that are presumably more reactive are present at lower apparent concentrations in the cytoplasm based on our FRET measurements.
DISCUSSION
Due to decades of human activities, large amounts of heavy metals have been released or have leached into the environment. Currently, chemical- and engineering-based processes are commonly employed for heavy metal containment, including the capping of contaminated sediments for their removal. Recently, increased importance has been attached to bioremediation as an alternative method for removing pollutants from soil and water bodies. Due to the high metal-binding capacity of algae, they have been at the forefront of toxic heavy metal bioremediation efforts in aquatic systems (Cai et al., 1995, 1999; Adhiya et al., 2002; Mehta and Gaur, 2005; Tüzün et al., 2005). For example, it has been shown that C. reinhardtii can adsorb toxic metal loads equal to one-third of the dry weight of the cell and that genetically engineered organisms expressing surface-exposed MT have greatly improved metal-binding capacity (Cai et al., 1995, 1999; Adhiya et al., 2002; Siripornadulsil et al., 2002; Mehta and Gaur, 2005; Tüzün et al., 2005). Although quantitative evidence relating to the cell wall-associated toxic metal complexation of Pb2+, Cd2+, and Cu2+ is available (Adhiya et al., 2002; Tüzün et al., 2005), until this study, there have been no in vivo measurements of cytosolic free (noncomplexed) toxic metals in algae. In determining cytosolic toxic metal concentrations, we successfully developed an MT-FRET biosensor system (Figs. 1 and 4). As discussed previously, C. reinhardtii cell walls sequester toxic metals as complexes to amino, carboxyl, hydroxyl, and carbonyl groups (Adhiya et al., 2002; Tüzün et al., 2005). To reduce metal sequestration by cell wall components, a transgenic cell wall-deficient C. reinhardtii strain, a cell wall-defective mutant cw15 derivative (He et al., 2011), was used for the expression of the CMY biosensor in the cytosol. This system presumably allowed for the direct and rapid transfer of metals across the plasma membrane without having to cross the cell wall barrier, which binds and limits the metal accessibility. As shown in Figure 5, the addition of MT-II-binding heavy metals such as cadmium resulted in an immediate FRET response in CMY transgenic cells. Significantly, the detectable internal toxic metal concentrations were severalfold (6- to 25-fold) lower than the external concentrations (CMY saturating) for the heavy metals lead and cadmium.
There are several possible reasons for the reduced internal metal concentrations relative to the external concentrations. While cw-15 cell wall-less mutant strains presumably have greater cytoplasmic metal accessibility than walled, wild-type strains, they still have some cell wall material, which might sequester some fraction of the added metals. Even with such limitations, based on the FRET response kinetics, the uptake/transport and equilibration of metal appear to be rapid (less than 1 s) in the algal cytosol (Fig. 5). This observation also indicates that metal transport kinetics do not limit metal accumulation in the cytoplasm during the time course of these experiments. However, heavy metals do form tight complexes with cytoplasmic organic compounds such as glutathione and organic acids and are effectively buffered by these molecules (Siripornadulsil et al., 2002), potentially impacting biosensor response. Importantly, given the short time scale over which our experiments were conducted, cellular adaptive metal detoxification mechanisms such as phytochelatin synthesis would not be expected to contribute to the sequestration of heavy metals (Howe and Merchant, 1992; Hu et al., 2001). Under short time periods, heavy metal complexation by reduced glutathione and organic acids probably contributes substantially to metal detoxification. Consistent with this hypothesis was the observation that apparent cytoplasmic concentrations of MT-II binding metals based on FRET responses were lowest for the most chemically reactive metals. Since both essential and toxic metals are well buffered in the cytoplasm, our results indicate that metal homeostasis in the cytoplasm is likely to be complex due to the large diversity of functional groups capable of binding metals in cells. It is also suggested that the metal-buffering capacity of cells provides a substantial metal reservoir for the cell.
MATERIALS AND METHODS
DNA Manipulation and Plasmid Constructs
A gene cassette containing codon-optimized Chlamydomonas reinhardtii and color-shifted variants (CFP and YFP) of the gene encoding the jellyfish GFP (Tsien, 1998; Fuhrmann et al., 1999; Zacharias et al., 2002) were cloned into the bacterial expression vector pMALc2X (New England Biolabs) as an N-terminal MBP fusion protein. A chicken MT-II complementary DNA (National Center for Biotechnology Information accession no. X06749) was inserted in frame between the CFP- and YFP-encoding genes to make the fusion protein (CMY). This bacterial expression system, designated pMALc2X-CMY, was utilized for overproduction of the MBP-CMY fusion protein. An algal nuclear transformation vector for cytosolic CMY expression was generated by subcloning the CMY fragment (5′ KpnI/3′ PstI) into pSSCR7 (Siripornadulsil et al., 2002) digested with the same restriction enzymes, creating plasmid pSSCR7-CMY.
CMY Expression in Escherichia coli and in Vitro Metal-Binding Studies
E. coli ER2508 (New England Biolabs) transformed with pMALc2X-CMY was grown at 28°C in Luria-Bertani broth supplemented with 100 mg L−1 ampicillin. Protein expression was induced with 0.25 mm isopropyl thiogalactoside at a cell density (optical density at 600 nm) of 0.6, and cells were grown for an additional 5.5 h. Bacterial cells were harvested by centrifugation. All protein purification steps were carried out at 4°C. Cells were resuspended and sonicated in 50 mm HEPES (pH 7.4) and 100 mm NaCl (buffer A) with 10 mm 2-mercaptoethanol (Sigma), 10 mm EDTA, and 1.0 mm phenylmethylsulfonyl fluoride (Affymetrix) to release the MBP-CMY protein. The clarified cell lysate was loaded onto an amylose affinity resin (NEB) equilibrated with buffer A and washed extensively. The bound MBP-CMY protein was eluted with 50 mm HEPES (pH 7.4), 100 mm NaCl, and 10 mm maltose (buffer B). To remove MBP from the fusion protein, the purified MBP-CMY (3.4 mg mL−1) was incubated with 10 µg of factor Xa supplemented with 1.0 mm CaCl2 (37 h, 4°C). The digestion mixture containing MBP, CMY, and MBP-CMY protein was buffer exchanged with buffer A using a Sephadex G-25 (GE Healthcare Bio-Sciences) size-exclusion column (60 cm × 1.5 cm). The CMY was separated from MBP and MBP-CMY by passing through regenerated amylose resin and collecting the flow through containing the CMY fusion protein only. The purified CMY was further incubated with 15 mm 2-mercaptoethanol for 16 h at 4°C. CMY preparations used for metal-binding studies were carried out using degassed reagents. Immediately before metal-binding studies, 2-mercaptoethanol was removed by chromatography with buffer A on a Sephadex G-25 size-exclusion column as detailed above. Protein purity was evaluated by SDS-PAGE, and the protein yield was determined using a Bradford protein assay kit (Bio-Rad). Metal-binding studies were carried out by adding CMY protein to a final concentration of 325 nm to aliquots of different concentrations of metal solutions in 50 mm HEPES, pH 7.0. The metal salts used for FRET studies included CdCl2 (1.0 m), CuSO40·5H2O (1.0 m), ZnCl2 or ZnSO4 (1.0 m), HgCl2 (0.2 m), MgCl2 (1.0 m), and Na2SO4 or NaCl (1.0 m). The final NaCl concentration for all metal-binding studies was maintained at 6.4 mm. CFP and YFP fluorescence measurements were carried out immediately after the addition of the biosensor to buffered metal solutions. All fluorescence measurements were made at λex 440 nm (slit 5 nm) and λem 460 to 560 nm (slit 5 nm) using a Cary Eclipse spectrofluorometer (Varian).
Algal Strains and Culture Conditions
The C. reinhardtii cell wall-less strain CC-425 (arg7-8 cw15 sr-u-2-60 mt+) was grown on TAP (Harris, 1989) agar supplemented with 100 mg L−1 Arg (Sigma) and 50 mg L−1 ampicillin. The CMY transgenic algae were maintained on TAP agar without Arg. For liquid cultures, cells were grown in modified TAP medium (glycerophosphate TAP [gTAP]) in which the inorganic phosphate (1.0 mm) was substituted with 1.0 mm glycerophosphate (Collard and Matagne, 1990). The gTAP algal liquid cultures were grown with ampicillin (50 mg L−1) under continuous illumination (75 µmol m−2 s−1) and shaking (150 rpm).
C. reinhardtii Transformation and in Vivo Metal Studies
Nuclear cotransformation of C. reinhardtii strain CC-425 was carried out with CMY-expressing plasmid construct pSSCR7-CMY and with the Arg-7-encoding plasmid p389 by glass-bead (Kindle, 1990) and electroporation (Shimogawara et al., 1998) transformation methods. For nuclear transformation, linearized vectors, 3.0 µg of pSSCR7-CMY (SspI) and 1.5 µg of p389 (EcoRV), were used with 300 µL (approximately 108 cells mL−1) of algae prepared as follows. C. reinhardtii cells were grown in 100 mL of TAP to a cell density of 1 to 2 × 106 cells mL−1, harvested by centrifugation (6,000g, 5 min), and resuspended in 1.0 mL of TAP + 40 mm Suc. To 300 µL of algal cells, 300 mg of 0.425- to 0.6-mm sterilized glass beads (Sigma) and DNA were added, vortexed (20 s), and plated on TAP plates. The transformants were selected on TAP agar medium for Arg auxotrophy. For electroporation transformation, linearized vectors, pSSCR7-CMY (SspI) and p389 (EcoRV), were used with 250 µL of algae prepared as follows. The TAP medium-grown culture was chilled on ice, and 50 µL of 10% (v/v) Tween 20 was added. The cells were harvested by centrifugation (800g, 5 min at 4°C), and the pellet was resuspended in 1.0 mL of TAP + 40 mm Suc. In a 4-mm electroporation cuvette, 250 µL of cells plus linearized vectors containing 5.0 µg of transgene (pSSCR7-CMY), 2 µg of selectable marker DNA (p389), and 50 µg of salmon sperm DNA (Invitrogen) were incubated at 20°C for 5 min. An electroporation pulse of 2,400 V cm−1 with capacitance at 10 µF and time constant of 5 ms was applied using a MicroPulsor (Bio-Rad). The algal transformants were selected on TAP agar plates for growth in the absence of Arg.
Putative transformants were utilized for genomic DNA isolation using a modified xanthogenate mini-prep method described by Dr. Steve Surzycki (http://www.chlamy.org/methods/dna.html). Half a loop of putative transgenic algae was resuspended in 300 µL of xanthogenate buffer (12.5 mm potassium ethyl xanthogenete, 100 mm Tris-HCl, pH 7.5, 80 mm EDTA, pH 8.5, and 700 mm NaCl) and incubated at 65°C for 1.0 h. Following incubation, the cell suspension was centrifuged for 10 min (16,000g), and the supernatant was collected. To the supernatant was added 2.5 volume of 95% (v/v) ethanol (750 µL) followed by inverting the tube several times. The ethanol-containing mixture was centrifuged for 5 min (16,000g), and DNA was collected by discarding the ethanol. The DNA pellet was washed with 700 µL of ice-cold 70% (v/v) ethanol, and centrifugation was repeated for 3.0 min. The ethanol was removed completely by decanting, and the rest was removed using a Kimwipe as a wick. The DNA pellet was resuspended in 100 µL of 10 mm Tris-HCl, pH 8.0. A small volume (5.0 µL) of the DNA preparation was used as a template in 50-µL PCR. Transgenics were identified using CMY gene fusion-specific PCR with isolated genomic DNA and the 5′-CACTACCAGCAGAACACCCCCATCGGCGATGGCCCCGTG-3′ and 5′-ACCGGTGAACAGCTCCTCGCCCTTGGC-3′ primer set, with plasmid pSSCR7-CMY as a positive control. The PCR-amplified gene products were also sequenced to further confirm the DNA sequence. PCR-positive transgenics and untransformed C. reinhardtii were examined using an E600 fluorescence microscope (Nikon Instruments) for the presence of cyan or yellow fluorescence. CMY fluorescence was visualized with a filter set consisting of an excitation filter of 450 to 490 nm, a dichroic mirror of 510 nm, and a barrier filter of 520 to 560 nm.
Wild-type (CC-2137) and CMY-expressing cells were grown in TAP or gTAP, and the cell density was determined by light scattering at 750 nm using a Cary UV-Vis spectrophotometer (Varian). For metal-binding studies, cells grown in gTAP were collected in the exponential growth phase (optical density at 750 nm of 0.8–1.0). Cell counts were routinely carried out using a hemocytometer. In vivo biosensor fluorescence measurements were recorded with a spectrofluorometer using measurement parameters described in the figure legends.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. FRET Heavy metal biosensor model.
Supplemental Figure S2. Fluorescence emission image of CMY FRET transgenic and wild-type algae.
Supplemental Figure S3. Fluorescence emission profiles of Chlamydomonas wild-type cells.
Supplemental Table S1. Metal speciation analysis.
Acknowledgments
We thank Dr. Biao Ding for fluorescence microscopy.
Glossary
- MT
metallothionein
- FRET
fluorescence resonance energy transfer
- CFP
cyan fluorescent protein
- YFP
yellow fluorescent protein
- CMY
N-terminal cyan fluorescent protein linked to MT-II and a C-terminal yellow fluorescent protein
- λex
maximum emission wavelength
- TAP
Tris-acetate-phosphate
- gTAP
glycerophosphate Tris-acetate-phosphate
References
- Adhiya J, Cai X, Sayre RT, Traina SJ. (2002) Binding of aqueous cadmium by the lyophilized biomass of Chlamydomonas reinhardtii. Colloids Surf A Physicochem Eng Asp 210: 1–11 [Google Scholar]
- Cai XH, Brown C, Adihya C, Traina S, Sayre RT. (1999) Growth and heavy metal binding properties of transgenic algae (Chlamydomonas reinhardtii) expressing a foreign metallothionein gene. International Journal of Phytoremediation 1: 53–65 [Google Scholar]
- Cai XH, Logan T, Gustafson T, Traina S, Sayre RT. (1995) Applications of eukaryotic algae for the removal of heavy metals from water. Mol Mar Biol Biotechnol 4: 338–344 [Google Scholar]
- Cobbett C, Goldsbrough P. (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53: 159–182 [DOI] [PubMed] [Google Scholar]
- Collard JM, Matagne RF. (1990) Isolation and genetic analysis of Chlamydomonas reinhardtii strains resistant to cadmium. Appl Environ Microbiol 56: 2051–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann M, Oertel W, Hegemann P. (1999) A synthetic gene coding for the green fluorescent protein (GFP) is a versatile reporter in Chlamydomonas reinhardtii. Plant J 19: 353–361 [DOI] [PubMed] [Google Scholar]
- Hanikenne M, Krämer U, Demoulin V, Baurain D. (2005) A comparative inventory of metal transporters in the green alga Chlamydomonas reinhardtii and the red alga Cyanidioschizon merolae. Plant Physiol 137: 428–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EH (1989) The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. Academic Press, San Diego [DOI] [PubMed] [Google Scholar]
- He Z, Siripornadulsil S, Sayre RT, Traina SJ, Weavers LK. (2011) Removal of mercury from sediment by ultrasound combined with biomass (transgenic Chlamydomonas reinhardtii). Chemosphere 83: 1249–1254 [DOI] [PubMed] [Google Scholar]
- Hill KL, Hassett R, Kosman D, Merchant S. (1996) Regulated copper uptake in Chlamydomonas reinhardtii in response to copper availability. Plant Physiol 112: 697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SH, Maret W. (2003) A fluorescence resonance energy transfer sensor for the beta-domain of metallothionein. Proc Natl Acad Sci USA 100: 2255–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe G, Merchant S. (1992) Heavy metal-activated synthesis of peptides in Chlamydomonas reinhardtii. Plant Physiol 98: 127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Lau KW, Wu M. (2001) Cadmium sequestration in Chlamydomonas reinhardtii. Plant Sci 161: 987–996 [Google Scholar]
- Kägi JH. (1991) Overview of metallothionein. Methods Enzymol 205: 613–626 [DOI] [PubMed] [Google Scholar]
- Kindle KL. (1990) High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 87: 1228–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looger LL, Lalonde S, Frommer WB. (2005) Genetically encoded FRET sensors for visualizing metabolites with subcellular resolution in living cells. Plant Physiol 138: 555–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SK, Gaur JP. (2005) Use of algae for removing heavy metal ions from wastewater: progress and prospects. Crit Rev Biotechnol 25: 113–152 [DOI] [PubMed] [Google Scholar]
- Merchant SS, Allen MD, Kropat J, Moseley JL, Long JC, Tottey S, Terauchi AM. (2006) Between a rock and a hard place: trace element nutrition in Chlamydomonas. Biochim Biophys Acta 1763: 578–594 [DOI] [PubMed] [Google Scholar]
- Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. (2002) A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol 20: 87–90 [DOI] [PubMed] [Google Scholar]
- Pearce LL, Gandley RE, Han W, Wasserloos K, Stitt M, Kanai AJ, McLaughlin MK, Pitt BR, Levitan ES. (2000) Role of metallothionein in nitric oxide signaling as revealed by a green fluorescent fusion protein. Proc Natl Acad Sci USA 97: 477–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarshani I, Sahu D, Rath B. (2011) Microalgal bioremediation: current practices and perspectives. J Biochem Tech 3: 299–304 [Google Scholar]
- Rauser WE. (1990) Phytochelatins. Annu Rev Biochem 59: 61–86 [DOI] [PubMed] [Google Scholar]
- Romero-Isart N, Vasák M. (2002) Advances in the structure and chemistry of metallothioneins. J Inorg Biochem 88: 388–396 [DOI] [PubMed] [Google Scholar]
- Selvin PR. (1995) Fluorescence resonance energy transfer. Methods Enzymol 246: 300–334 [DOI] [PubMed] [Google Scholar]
- Shimogawara K, Fujiwara S, Grossman A, Usuda H. (1998) High-efficiency transformation of Chlamydomonas reinhardtii by electroporation. Genetics 148: 1821–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siripornadulsil S, Traina S, Verma DP, Sayre RT. (2002) Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 14: 2837–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki KT, Maitani T. (1981) Metal-dependent properties of metallothionein: replacement in vitro of zinc in zinc-thionein with copper. Biochem J 199: 289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RY. (1998) The green fluorescent protein. Annu Rev Biochem 67: 509–544 [DOI] [PubMed] [Google Scholar]
- Tüzün I, Bayramoğlu G, Yalçin E, Başaran G, Celik G, Arica MY. (2005) Equilibrium and kinetic studies on biosorption of Hg(II), Cd(II) and Pb(II) ions onto microalgae Chlamydomonas reinhardtii. J Environ Manage 77: 85–92 [DOI] [PubMed] [Google Scholar]
- Vasák M. (2005) Advances in metallothionein structure and functions. J Trace Elem Med Biol 19: 13–17 [DOI] [PubMed] [Google Scholar]
- Weiss S. (2000) Measuring conformational dynamics of biomolecules by single molecule fluorescence spectroscopy. Nat Struct Biol 7: 724–729 [DOI] [PubMed] [Google Scholar]
- Zacharias DA, Violin JD, Newton AC, Tsien RY. (2002) Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296: 913–916 [DOI] [PubMed] [Google Scholar]
- Zimmer M. (2002) Green fluorescent protein (GFP): applications, structure, and related photophysical behavior. Chem Rev 102: 759–781 [DOI] [PubMed] [Google Scholar]