Abstract
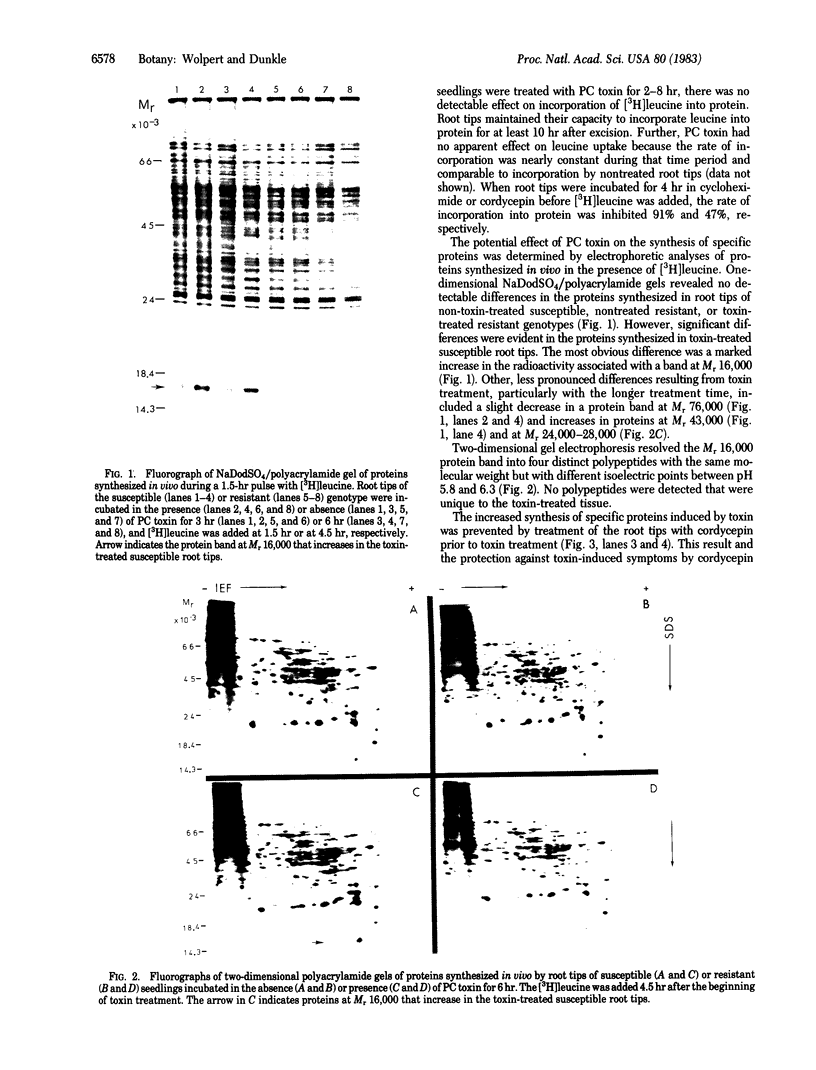
Susceptibility of sorghum to the fungal pathogen Periconia circinata and sensitivity to its host-specific toxin are determined by the semidominant allele at the pc locus. Pretreatment of susceptible seedlings with cycloheximide or cordycepin for 4 hr before treatment with the toxin protected the seedlings against toxin-induced loss of electrolytes and prevented development of disease symptoms. In vivo incorporation of [3H]leucine into protein was inhibited 91% and 47% by cycloheximide and cordycepin, respectively, but was not affected by the toxin. Gel electrophoresis and fluorography of in vivo-labeled proteins extracted from non-treated and toxin-treated root tips of near-isogenic susceptible and resistant lines revealed a selective increase in radioactivity of a protein band at Mr 16,000 only in preparations from toxin-treated susceptible root tips. Two-dimensional gel electrophoresis separated the Mr 16,000 band into four proteins and confirmed the increased rate of synthesis. Products of in vitro translation were substantially enriched with the four Mr 16,000 proteins when total RNA from toxin-treated susceptible root tips was used in a cell-free protein-synthesizing system. Because the proteins that increase are common to both susceptible and resistant genotypes, the toxin apparently interferes with a regulatory function, perhaps a function of the pc locus, and thereby alters gene expression in the susceptible genotype. The data suggest but do not establish that phytotoxicity results from the increased rate of synthesis of the specific proteins.
Keywords: pathogenesis, phytotoxin, milo disease, protein synthesis
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Filner P., Varner J. E. A test for de novo synthesis of enzymes: density labeling with H2O18 of barley alpha-amylase induced by gibberellic acid. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1520–1526. doi: 10.1073/pnas.58.4.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. T., Varner J. E. Hormonal control of messenger ribonucleic acid metabolism in barley aleurone layers. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4783–4786. doi: 10.1073/pnas.71.12.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L. Ribonucleic Acid and Protein Synthesis as Essential Processes for Cell Elongation. Plant Physiol. 1964 May;39(3):365–370. doi: 10.1104/pp.39.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Hurkman W. J. Synthesis and deposition of zein in protein bodies of maize endosperm. Plant Physiol. 1978 Aug;62(2):256–263. doi: 10.1104/pp.62.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizardi P. M., Engelberg A. Rapid isolation of RNA using proteinase K and sodium perchlorate. Anal Biochem. 1979 Sep 15;98(1):116–122. doi: 10.1016/0003-2697(79)90714-0. [DOI] [PubMed] [Google Scholar]
- Mozer T. J. Control of protein synthesis in barley aleurone layers by the plant hormones gibberellic acid and abscisic acid. Cell. 1980 Jun;20(2):479–485. doi: 10.1016/0092-8674(80)90634-0. [DOI] [PubMed] [Google Scholar]
- Mozer T. J. Partial purification and characterization of the mRNA for alpha-amylase from barley aleurone layers. Plant Physiol. 1980 May;65(5):834–837. doi: 10.1104/pp.65.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noodén L. D., Thimann K. V. EVIDENCE FOR A REQUIREMENT FOR PROTEIN SYNTHESIS FOR AUXIN-INDUCED CELL ENLARGEMENT. Proc Natl Acad Sci U S A. 1963 Aug;50(2):194–200. doi: 10.1073/pnas.50.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rayson B. M., Carney S., Gardner G., Morgan T. The interrelationships between antidiuretic hormone, adenyl cyclase, tissue cyclic AMP and diffusional water permeability. Clin Exp Pharmacol Physiol. 1976 Mar-Apr;3(2):147–157. doi: 10.1111/j.1440-1681.1976.tb00599.x. [DOI] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Walker J. C., Key J. L. Isolation of cloned cDNAs to auxin-responsive poly(A)RNAs of elongating soybean hypocotyl. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7185–7189. doi: 10.1073/pnas.79.23.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcockson J. The differential precipition of nucleic acids and proteins from aqueous solutions by ethanol. Anal Biochem. 1975 May 26;66(1):64–68. doi: 10.1016/0003-2697(75)90724-1. [DOI] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin- and ethylene-induced changes in the population of translatable messenger RNA in Basal sections and intact soybean hypocotyl. Plant Physiol. 1982 Feb;69(2):338–340. doi: 10.1104/pp.69.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin-induced changes in the patterns of protein synthesis in soybean hypocotyl. Proc Natl Acad Sci U S A. 1980 Jan;77(1):357–361. doi: 10.1073/pnas.77.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]