Abstract
Dupuytren's disease (DD) is a benign fibroproliferative disease of the hand. It is characterized by the excessive production of extracellular matrix (ECM) proteins, which form a strong fibrous tissue between the handpalm and fingers, permanently disrupting the fine movement ability. The major contractile element in DD is the myofibroblast (MFB). This cell has both fibroblast and smooth muscle cell-type characteristics and causes pathological collagen deposition. MFBs generate contractile forces that are transmitted to the surrounding collagen matrix. Μajor profibrotic factors are members of the transforming growth factor-β (TGFβ) pathway which directly regulate the expression levels of several fibrous proteins such as collagen type 1, type 3, and α-smooth muscle actin. Molecular modulation of this signaling pathway could serve as a therapeutic approach. We, therefore, have developed an ex vivo “clinical trial” system to study the properties of intact, patient-derived resection specimens. In these culture conditions, Dupuytren's tissue retains its three-dimensional (3D) structure and viability. As a novel antifibrotic therapeutic approach, we targeted TGFβ type 1 receptor (also termed activin receptor-like kinase 5) expression in cultured Dupuytren's specimens by antisense oligonucleotide-mediated exon skipping. Antisense oligonucleotides targeting activin receptor-like kinase 5 showed specific reduction of ECM and potential for clinical application.
Keywords: Dupuytren's, exon skipping, fibrosis, TGFβ
Introduction
Dupuytren's disease (DD) is a common fibrotic disorder of the hand, found with high prevalence among Caucasians of Northern European descent.1 This enigmatic benign fibroproliferative disease affecting the connective tissue (Figure 1a) results from a complex interplay of genetic, anatomic, and environmental factors2 with main clinical manifestation being the excessive collagen deposition. A disturbance of the heterogeneous mix of static and dynamic contractile elements located throughout the fascia of the palm and digits can lead to the development of flexion deformities (contracture). Although not associated with high morbidity, the impact on movement ability and quality of life of the patients affected by DD is major. Currently, the common therapy for DD is palmar fasciectomy, which consists of surgical removal of fibrotic tissue and results in immediate improvement of disease. However, due to the high recurrence rate and remanifestation of fibrotic bands, surgery is not a permanent solution.
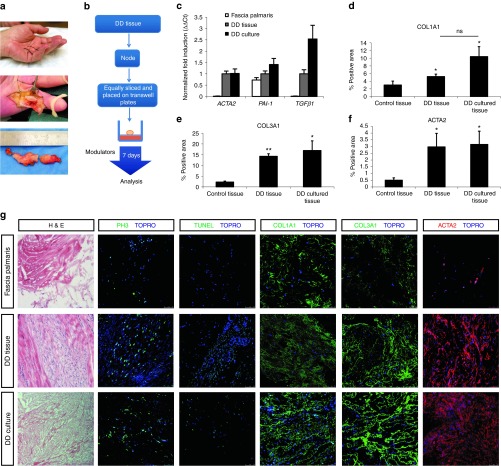
Figure 1.
Characterization of Dupuytren's disease (DD) contracture tissue in the three-dimensional (3D) ex vivo “clinical trial” system. (a) DD contracture in the hand of a patient; example of DD tissue prior and after resection. (b) Cartoon describing 3D culture method: DD tissues are equally sliced (about 1 mm), placed on a nitrocellulose membrane and cultured up to 7 days. (c) Quantitative polymerase chain reaction on control normal fascia palmaris (N = 7), DD noncultured tissue (see DD tissue, N = 4) and DD cultured tissue (see DD culture, N = 9). Error bars represent ±SEM. ACTA2, TGFβ1, and PAI-1 mRNA levels have been quantified and normalized to ACTRT1. Fold induction values compared to DD noncultured tissue are shown. (d–f) Quantification (described in methods) of immunofluorescence signal of COL1A1, COL3A1, and ACTA2 in normal fascia palmaris (N = 7), DD noncultured tissue (see DD tissue, N = 4), and DD tissue after 7 days 3D culture (see DD culture, N = 9). Multiple focal planes were quantified per sample, error bars represent ±SEM. Statistical significance was calculated by one-tailed paired t-test. *P < 0.05, **P < 0.01. (g) Immunohistochemical and immunofluorescent analysis of normal fascia palmaris, DD noncultured tissue, and DD cultured tissue. Hematoxylin and eosin (H&E), proliferation marker phosphohistone 3, green, apoptosis terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (green), Collagen type 1 (COL1A1, green), Collagen type 3 (COL3A1, green), and smooth muscle actin, α2 (ACTA2, red). Nuclei were visualized with TO-PRO3 (TOPRO, blue). Scale bars, 25 µm.
Several studies have elucidated the aetiopathology of DD which is crucial for the design of novel therapies. Uncontrolled wound healing response leads to permanent extracellular matrix (ECM) deposition, e.g., collagen. The cell responsible for ECM production in normal as well as in pathologic conditions is the myofibroblast (MFB) containing both fibroblast and smooth muscle cell-type characteristics.3 MFBs generate contractile forces that are transmitted to the surrounding collagen matrix4 and are distinguished by α-smooth muscle actin (ACTA2) expression. In pathological conditions, ACTA2 expression is persistent.
Μajor profibrotic factors are members of the transforming growth factor-β (TGFβ) pathway, which directly regulate the expression levels of several intracellular and extracellular fibrous proteins such as COL1A1, COL3A1, and ACTA2,3,5,6,7 fibronectin, matrix metalloproteases, and integrins, all of which are aberrantly deregulated in DD.8,9 TGFβ ligands interact with TGFβ type 1 receptor (also termed activin receptor-like kinase 5 (ALK5)/TGFβRI and type 2 (TGFβRII) receptor complexes which subsequently activate by phosphorylation the SMAD2/3, which form heteromeric complexes with SMAD4 and act as downstream transcriptional effectors of the pathway. Activation and transdifferentiation of DD fibroblasts toward MFBs is mainly controlled by TGFβ signaling.10,11,12,13 Other cytokines, such as platelet-derived growth factor, are induced by TGFβ14 and also enhance MFB differentiation. TGFβ and platelet-derived growth factor factors are aberrantly activated in DD.10,15,16 In particular, in DD patient-derived MFB cultures, overactive TGFβ signaling causes spontaneous contraction and proliferation.13,15 Contractility is attenuated by inhibiting TGFβ and TGFβ receptor (ALK5) function.15,17,18 We have recently shown that the TGFβ/SMAD and platelet-derived growth factor/ERK1/2 MAP kinase pathways cooperate in mediating the enhanced proliferation and spontaneous contraction of DD fibroblasts.15 Inhibiting the uncontrolled fibrotic mechanisms by directly targeting the overactivation of the TGFβ signaling, mediated via its ALK5 receptor, at the molecular level, could be an effective treatment.
A promising approach to deplete the cells from the function of key receptor of TGFβ signaling (ALK5) is by alternative splicing methodology. Particular exon(s) encoding protein domains crucial for protein function can become excluded from the mature messenger RNA (mRNA). Specific antisense oligonucleotides (AON) bind to sites involved in exon splicing to the splice sites of a targeted exon and interfere with the splice machinery; therefore, the particular exon is not integrated as part of the mRNA.19 The resulting mRNA has an intact open reading frame and is translated into a protein which lacks only the particular peptide sequence encoded by the skipped exon. The advantage of this system is that no genetic alterations are introduced, since interference is exclusively with pre-mRNA splicing process. AON methodology has broad therapeutic applicability in many human diseases, particularly in the field of muscular dystrophies20 with very promising results reported for clinical trials.21,22 Based on this principle, we employed the AON-mediated exon skipping technology for disrupting the protein function of the ALK5, targeting in particular the extracellular ligand-binding domain. AONs targeting splice sites of exon encoding extracellular ligand-binding domain (exon 2) of the ALK523 have been developed and tested in vivo (D.U. Kemaladewi et al, unpublished data). This strategy ensures no loss of other important domains of ALK5, such as the transmembrane domain (encoded by exon 3) or serine-threonine kinase activity domain (exon 4-9). ALK5 AON was administered directly to the DD patient-derived specimens by microinjecting it in the center of the tissue, and the effects on fibrosis and ECM deposition were assessed with various imaging and biochemical methods. In this study, we show that DD resected specimens, which are discarded as waste material after surgery, can be maintained viable in defined culture conditions in our novel ex vivo model. Their study can provide us with useful information about the underlying patient-specific pathology and drug response.
Results
Human-derived DD tissue can be maintained under ex vivo culture conditions
Fibroblast derivation from DD specimens requires a long culture period during which cells adapt to culture conditions (plastic surface, high oxygen, and removal of ECM). Such changes of the native microenvironment may result in partial recapitulation of the disease state or fibroproliferative characteristics of the tissue in fibroblast two-dimensional (3D) cultures.24,25 We have developed a 3D culture system (Figure 1a,b), which allows human resection specimens to be grown ex vivo (up to 7 days) in defined conditions. Longer culture periods (up to 12 days, data not shown) lead to increased cell death (cleaved caspase 3 positive cells) and absence of proliferation, suggesting nonviability of tissue after a certain time point (day 7). We show that DD resection specimens in the ex vivo “clinical trial” system maintain viability, proliferation (phosphohistone-3), and apoptosis levels (TUNEL) (Figure 1g). As control tissue, we have used normal fascia palmaris from carpal tunnel surgeries, which is not affected by DD. Control tissue was successfully maintained in culture for up to 7 days and is characterized by low levels of proliferation (phosphohistone-3) and apoptosis (TUNEL) (Supplementary Figure S1, upper panel). Histological characterization of the cultured DD biopsies showed that the high expression of fibrotic proteins: ACTA2, COL1A1, and COL3A1 is preserved (Figure 1g, representative images), therefore they recapitulate the in vivo properties. Similar data were obtained from a number of biopsies (normal fascia palmaris, N = 7, DD noncultured tissue, N = 4, and DD tissue after 7 days 3D culture, N = 9) indicating the reproducibility of the method. Quantification of immunofluorescence signal for COL1A1, COL3A1, and ACTA2 (Figure 1d–f), in multiple patient-derived specimens showed that biopsies cultured ex vivo retain the expression characteristics with regards to fibrosis.
Basal expression of ACTA2, COL1A1, and COL3A1 (Figure 1c–g), as well as TGFβ1 and PAI-1 mRNA levels (TGFβ target genes) (Figure 1c), are elevated in both cultured and noncultured DD resection specimens compared to fascia palmaris (control, nonaffected tissue). Moreover, the snap-frozen and the 3D cultured DD (matching) resection specimens similarly show areas of proliferating MFBs and low apoptosis (Figure 1g). All together, the above data indicate that DD tissue under culture conditions remains representative of the disease.
Small molecule inhibitor of TGFβ type 1 receptor kinase (SB-431542) decreases expression of fibrotic proteins in DD specimens
Our novel ex vivo culture method was further used to test the response of the DD tissue to stimulation with different factors directly after fasciectomy procedure. Main profibrogenic stimulus in DD is the TGFβ signaling; consequently, we decided to interfere with the activation status of this particular pathway. Resection specimens (both control (Supplementary Figure S1) and DD (Figure 2a) were treated with TGFβ ligand as well as a pharmacological TGFβ type 1 receptor (ALK4, ALK5, ALK7) kinase activity inhibitor (SB-431542). Addition of TGFβ to cultured DD specimens resulted in increased expression of target genes ACTA2, COL1A1, and COL3A1 in the majority of individual human samples tested or sustained the high levels (Figure 2b–d). This observation suggests high sensitivity of DD MFB cells to TGFβ, also confirmed by high expression of phosphorylated SMAD2 protein (pSMAD2) (Figure 2a). As expected, treatment with the SB-431542 inhibitor compound in our model suppressed the profibrogenic action of TGFβ and resulted in a trend reduction of the expression of fibrous proteins ACTA2, COL1A1, and COL3A1 (Figure 2). Differential expression levels among individual samples after TGFβ and/ or SB-431542 treatments were observed. Proliferation and apoptosis were not significantly affected by the addition of either TGFβ or SB-431542 (Figure 2a). Treatment of control tissue with TGFβ cytokine caused an upregulation of ACTA2, COL1A1, and COL3A1 expression (Supplementary Figure S1, middle panel), suggesting a responsiveness of the tissue to the treatment and underlining the profibrotic effect of TGFβ. The above observations may suggest that the 3D ex vivo culture system is suitable for chemical compound screening. Differences in the response of human specimens to growth factor or inhibitor SB-431542 most probably derives from variation among different individuals which can be effectively observed and represented using our ex vivo culture system.
Figure 2.
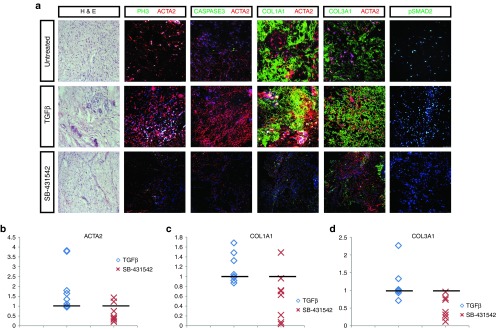
Inhibition and stimulation of transforming growth factor-β (TGFβ) pathway in Dupuytren's disease (DD) resection specimens cultured in three-dimensional (3D) ex vivo “clinical trial” system. (a) Immunohistochemical and immunofluorescent analysis of 3D cultured DD resection specimens before treatment (untreated) and after 7-day treatment with TGFβ and ALK4/5/7 kinase inhibitor SB-431542. Hematoxylin and eosin (H&E), proliferation marker phosphohistone 3 (PH3, green), apoptosis marker cleaved caspase 3 (CASPASE3, green), collagen type 1 (COL1A1, green), collagen type 3 (COL3A1, green), smooth muscle actin, α2 (ACTA2, red), and phosphorylated SMAD2 (pSMAD2) (green). Nuclei were visualized with TO-PRO-3 (TOPRO, blue). Representative data from eight patient-derived specimens (N = 8). Scale bars, 25 µm. (b–d) Distribution of fold quantitative values of (b) ACTA2, (c) COL1A1, (d) and COL3A1 among eight patient-derived specimens after ex vivo culture. Quantification of fluorescent signal within certain area fraction was calculated in Image J software for every specimen in three different conditions; untreated (control, no exogenous factors), TGFβ cytokine and ALK4/5/7 kinase inhibitor SB-431542 compounds. Graph represents the values of positive signal of ACTA2, COL1A1, COL3A1, after treatment with TGFβ or SB-431542 compounds as a fold induction over the value of the control “untreated” sample (indicated by black line).
AON-mediated exon skipping of the ALK5
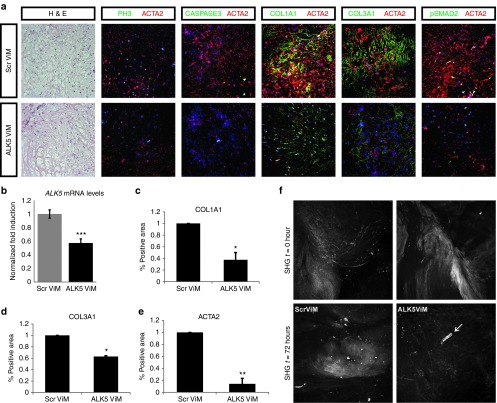
While treatment with SB-431542 resulted in a promising downregulation of fibrotic pathways, this chemical inhibitor blocks the kinase activity of ALK4, ALK5, and ALK7 in a dose-dependent way.26 Thus, in order to ensure more specificity and less interference with other signaling pathways, we have tested a novel strategy to selectively inhibit the function of the ALK5. We used vivo-morpholinos (ViM) based on previously developed AON sequence (D.U. Kemaladewi et al., unpublished data) that selectively target and disrupt the ligand-binding domain of ALK5 by inducing exon skipping of mRNA transcripts. Microinjection (Figure 3a) of resection specimens with fluorescently labeled AON demonstrated efficient uptake (>90%) and transport to the nucleus throughout the tissue (Figure 3b,c). Similarly, the AON targeting ALK5 (ALK5ViM) was microinjected in the centre of the tissues after placing them on the nitrocellular membrane of the transwell culture plates (Figure 3a). At day 3, we validated the skipping of exon 2 by PCR (Figure 3d,e) and verified reduction of full length ALK5 mRNA expression (Figure 4b) compared to tissues injected with control scrambled ViM (ScrViM). No effect on the proliferation rate and apoptosis was observed by the use of ALK5ViM (Figure 4a). We performed a time course experiment to monitor the levels of full length ALK5 mRNA expression versus the exon skipped mRNA. Full length ALK5 mRNA is decreased by 70–75% during the first 48 hours after AON administration (Supplementary Figure S2a). These data indicate that high rate of exon skipping is achieved at early time points and maintained in the tissue explant cultures. We also determined the collagen expression in different time points and observed a gradual decrease of COL1A1 expression (Supplementary Figure S2b).
Figure 3.
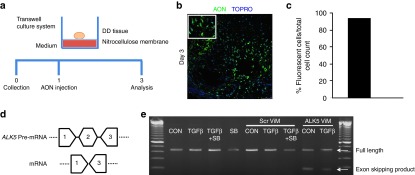
Microinjection of antisense oligonucleotides (AONs) in Dupuytren's disease (DD) resection specimen maintained in three-dimensional (3D) culture and ALK5 exon skipping. (a) AONs coupled to a fluorochrome (AON-fluorescent) were delivered by microinjecting the center of the tissue on the nitrocellulose membrane as described in the cartoon. The tissue was then cultured for 3 days and sectioned in order to determine the presence of nuclei, which had taken up the AON. (b) Direct visualization of the AON-fluorescent (green) and nuclei (TOPRO, blue) in a DD tissue section. (c) Quantification of the percentage of fluorescent cells (AON) relative to total cell count. Scale bars, 50 µm. (d) Description of exon skipping (exon 2) of the ALK5 pre-mRNA. Primer position (exon 1 and exon 3) of primers used for detecting the full length ALK5 and the exon skipped mRNA product are depicted here. (e) DD tissues were cultured and injected with either scrambled (ScrViM) or ALK5ViM. For comparison, treatment with transforming growth factor-β (TGFβ) or SB-431542 compound was also combined with ViM administration. After 3 days of treatments, tissues were homogenized and used for RNA isolation and cDNA synthesis. Touchdown PCR was performed to validate the exon skipping and products were visualized by agarose gel electrophoresis. Full length ALK5 mRNA transcripts were detected in all conditions while exon2-skipped ALK5 mRNA transcripts were only detected in tissues injected with ALK5ViM. CON: untreated condition; SB: treatment with SB-431542 compound; TGFβ: treatment with TGFβ cytokine; TGF+SB: treatment with both TGFβ and SB-431542 compound.
Figure 4.
ALK5ViM treatment of Dupuytren's disease (DD) resection specimens cultured in the three-dimensional (3D) ex vivo “clinical trial” system. (a) Immunohistochemical and immunofluorescent analysis of 3D cultured DD resection specimens after 3-day treatment with scrambled ViM (ScrViM) and ALK5ViM. Hematoxylin and eosin (H&E), proliferation marker phosphohistone 3 (PH3, green), apoptosis marker cleaved caspase 3 (CASPASE3, green), collagen type 1 (COL1A1, green), collagen type 3 (COL3A1, green), phosphorylated SMAD2 (pSMAD2) (green) and smooth muscle actin, α2 (ACTA2, red). Nuclei were visualized with TOPRO-3 (TOPRO, blue). (b) Quantitative polymerase chain reaction (Q-PCR) to detect expression levels of full length ALK5 mRNA was performed on tissues injected with ScrViM and ALK5ViM (N = 3). Values were normalized to CAPNS1. Fold induction values compared to ScrViM condition are shown. Statistical significance was calculated by one-tailed paired t-test. ***P < 0.001. (c–e) Quantification (described in methods) of immunofluorescence signal of COL1A1, COL3A1, and ACTA2 from different patient-derived specimens (N = 3) after 3-day treatment with scrambled ViM (ScrViM) and ALK5ViM. Fold induction values compared to ScrViM condition are shown. Multiple areas were quantified per sample, error bars represent ± SEM. Statistical significance was calculated by one-tailed paired t-test. *P < 0.05, **P < 0.01. Scale bars, 25 µm. (f) Second harmonic generation (SHG) images of endogenous DD tissue in the 3D culture system. Collagen distribution was imaged at control time point (SHG, t = 0, upper panel) in adjacent parts of the specimen. The exact tissue parts were imaged at 72 hours (SHG, t = 72 hours, bottom panel) after injection of ScrViM or ALK5ViM. Arrow indicates site of injection.
ALK5 AON causes a reversal of fibrotic phenotype ex vivo
Constant collagen deposition is the main feature of DD, thus, clinical attempts have been focused on direct induction of collagen degradation in vivo, such as by injectable collagenase treatment.27 Although very promising, this therapeutic approach is associated with several limitations (high morbidity) and cannot completely replace the surgical treatment.28
Our objective was to interfere with the fibrogenic role of TGFβ in a clinically relevant manner. However, TGFβ is a regulator of many crucial processes such as inflammation and would healing in many organs and is secreted by many cell types including macrophages, endothelial cells, lymphocytes, and epithelial cells. Thus, TGFβ should be tightly regulated and complete abolishment may lead to adverse effects. The process of exon skipping by AONs is advantageous because it results in partial and not complete blockage of the ALK5 receptor activity. Administration of the ALK5ViM directly to DD tissues (N = 3) remarkably reduced the overall protein expression of ACTA2, COL1A1, and COL3A1 (Figure 4a) and activation of downstream pSMAD2 (Figure 4a; Supplementary Figure S3), rendering the tissue more similar to the control, normal fascia palmaris (Supplementary Figure S1). Quantification of the expression patterns among different specimens (N = 3) confirmed a reproducible decrease observed after ALK5ViM treatment (Figure 4c–e). The spatiotemporal imaging of the endogenous extracellular distribution of collagen structure was determined by second harmonic generation on DD specimens during 3D culture, prior and after ALK5ViM application (Figure 4f). Reorganization/degradation of collagen fibers, specifically at the site of injection with the ALK5ViM was observed with second harmonic generation (Figure 4f, white arrow) similarly to immunofluorescence signal (Figure 4a). In contrast, tissue injected with ScrViM retained the highly anisotropic collagen structures and did not exhibit signs of reorganization/degradation (Figure 4f). A partial reduction of available ALK5 molecules appears already sufficient to reduce the fibroproliferative effect mediated by TGFβ in fibroblasts/MFBs. Importantly, mRNA molecules that escape exon skipping do produce functional ALK5 protein able for ligand binding (TGFβ) and protein complex formation with type 2 receptors. Thus, application of AONs does not cause complete abolishment of the TGFβ signaling, which is required at a basal level for tissue/ECM maintenance.
Discussion
In the present study, we have developed a novel method for ex vivo analysis of human DD disease and we provide evidence of its suitability for molecular modulation by AONs. AONs were designed to target and inhibit a key profibrotic signaling pathway, which results in significant antifibrotic effects. Given the high risk of recurrence of DD, it would be therapeutically beneficial to reduce local collagen content in order to extend the symptom-free period after surgery, needle fasciotomy, and/or collagenase injection. Our ex vivo “clinical trial” system allows the culture of DD specimens after surgical removal, without the need of fibroblast derivation, or grafting experiments,29 while preserving the pathological status of the disease by maintaining the complex organization of the ECM and the 3D tissue structure.
The main challenges in ex vivo culture methods are viability and preservation of the in vivo normal or pathological traits of the tissue to be studied. Several organ culture and precision cut tissue slice methods have been developed such as the submerged system,30 the dynamic organ culture,31 and the gas exchange method.30 Organ viability, functionality, metabolism, and toxicity can be well studied in all these systems for complex organs such as liver, kidney, intestine, and lungs.32,33 A limitation of these methods is the relatively short incubation time possible (~24–72 hours), depending on the tissue origin, as well as the challenge of organ/disease recapitulation. Our methodology is based on an enhanced setup where tissue parts are placed continuously and statically in contact with nutrients but are not fully immersed into medium, thus maintaining proper oxygenation and avoiding necrosis in the center of the tissue. Such setup appears suitable for culture of dense tissue such as DD fibrotic parts and facilitates viability for longer periods (up to 7 days tested). Exposure of one side of the tissue to the medium is sufficient for diffusion and absorbance of nutrients throughout the tissue. Small tissue parts (< 200 µm) are preferable in order to allow cell proliferation and longer viability.34 Static incubation was performed, in contrast to most dynamic culture conditions, in order to maintain positional information and cellular sensing.35,36 In addition, this particular setup allows for manipulation (e.g., AON injection) and direct visualization of the effects on the ECM (second harmonic generation). Since DD tissue shows rapid production of ECM proteins, all tissues were cultured in the absence of any exogenous matrix substrates. This is advantageous for the maintenance of native ECM turnover. Moreover, this culture setup is optimal for DD fibrotic tissue due to the content of highly proliferative MFBs and because the nodules and cords are in vivo quite isolated structures with autonomous characteristics (such as cell/ tissue growth and fibrosis). Due to these innate properties, it is likely that the tissues can be maintained ex vivo efficiently.
In this study, we have exclusively utilized the nodule parts, which are the firm thickenings and are considered pathologically very active due to the high content of MFBs. Cord parts are mainly fibrotic flexions and contain few fibroblasts, which are in a dormant state.37 It has been proposed that active nodules may progress into cord structures at more advanced stage of the disease;38 therefore, it is more clinically relevant to target the fibrotic characteristics of the node parts. TGFβ has been found to be expressed in both parts, as well as in the surrounding tissue4 (appearing not affected by the disease), which may play a role in promoting recurrence of fibrosis as part of wound healing response due to tissue damage from the primary surgery. The majority of the resection specimens we have analyzed using this system respond to TGFβ stimulation by upregulation or maintenance of the expression levels of fibrotic proteins (Figure 2b–d). Decrease in COL1A1 and COL3A1 but not of ACTA2 has been detected in two biopsies after TGFβ stimulation, which may suggest the function of a negative feedback loop due to high levels of TGFβ.39,40 Interestingly, when DD biopsies were treated with SB-431542 inhibitor, expression of collagen and ACTA2 was decreased in the majority of biopsies or sustained the same levels as if untreated (Figure 2b–d).
Previous studies have attempted manipulation of TGFβ by neutralizing antibodies29 and kinase inhibitors.15 TGFβ has been also targeted in indirect ways such as by cyclic AMP,41 angiotensin inhibitors,42 tamoxifen,43 and administration of bone morphogenetic protein-6.15 Given the pleiotropic effect of TGFβ signaling, the aim is to normalize and not completely abolish its function. Therefore, in order to restore the balance of pathway activation without fully disrupting its function, we have selectively inhibited the ALK5-mediated profibrotic pathway by exon skipping technology. It is worth noting that AON approach provides the advantage of high specificity exclusively for ALK5 mRNA (exon 2 encoding ligand-binding domain), while the SB-431542 compound targets activity of three kinase receptors (ALK4, ALK5, and ALK7), all implicated in the activin/TGFβ pathway. Moreover, SB-431542 may not block TGFβ/ALK5-induced non-SMAD signaling44,45 whereas ALK5 AON will inhibit both pathways. TGFβ/p38 and ERK MAP kinases have been shown to be involved in fibroproliferative response in DD.15 Delivery of ALK5 AON by affecting SMAD and non-SMAD TGFβ signaling may thus achieve better inhibition than ALK5 kinase inhibitors by interfering with multiple pathways downstream of ALK5. Compared to regular oligonucleotides, small molecule inhibitors have better pharmacokinetic properties, due to the short half-lives and inability to efficiently cross tissue membranes. However, currently, there are many oligonucleotide modifications available that ensure improved stability, serum half-life, and uptake of oligonucleotides. Our studies here use ViMs, which are antisense phosphorodiamidate morpholino oligomers covalently linked to a molecular scaffold that carries a guanidinium group at each of its eight tips to enhance delivery, to show proof-of-concept for this approach. Efficacy of ViMs has also been shown by others in animal models.46 However, further clinical development of this particular compound is hampered by toxic effects. Nevertheless, there is a plethora of chemical modifications available that can be studied further for clinical development.47 In light of this, it is encouraging that we were able to obtain similar results with ALK5 AONs of the 2′-O-methyl phosphorothioate AON chemistry (Supplementary Figure S4, which is very similar to the chemistry approved by US Food and Drug Administration (mipomersen)48 and identical to drisapersen, which is in phase 3 clinical trials for Duchenne muscular dystrophy). TGFβ secretion might also play a significant role in the recurrence of fibrosis after surgical removal. In this context, a hypothetical therapeutic setting would be the administration of AONs prior to or instead of the surgical intervention to counteract the TGFβ signaling in the remaining MFBs.
A challenge in the field of Dupuytren's is the lack of in vivo modeling of the disease. Here, we have developed a very robust and reproducible ex vivo 3D culture method with a simple setup (no growth factors or matrix protein support required). By using the ALK5ViM AON in this system, we have showed significant decrease in collagen protein expression and degradation/reorganization of collagen structures. Excessive collagen production is the main clinical symptom in this disease and here we provide proof of decrease in collagen deposition ex vivo. The average reduction of full length ALK5 mRNA achieved was 70–75% within the first 48 hours (Supplementary Figure S2a) and about of 30–60% by day 3 (Figure 4b; Supplementary Figure S2a). Our data indicate the potential of MFBs to reverse into a less fibrotic phenotype and to respond to growth factor inhibition even after advanced disease progression. In addition, we show the feasibility of a well-established ex vivo imaging approach, such as the second harmonic generation,49 for the study of ECM structure in native unstained tissue which to our knowledge has not been previously used for DD. The above observations may change the view of therapeutic approaches currently used for DD. Ultimately, the ex vivo “clinical trial” system can be applied for individualized therapy research after tissue resection as a drug screening method to test for specific responsiveness of DD tissues to a panel of growth factors and inhibitors and eventually lead to targeted therapy in case of recurrence.
Materials and methods
Generation of 3D culture system. Specimens from DD surgeries are equally sliced and placed in transwell plates onto 0.4 µm nitrocellulose membranes (Greiner Bio One, Alphen aan den Rijn, the Netherlands) in defined culture conditions (Dulbecco's modified Eagle's medium, with 1% fetal calf serum, 1% penicillin-streptomycin) and allowed to grow (7 days). Nutrient exchange occurs by diffusion from the medium through the membrane while DD tissue remains continuously in contact with the liquid but is not immerged. Tissue resection specimens (N = 9 DD and N = 4 normal fascia palmaris) were treated with a combination of activators and inhibitors of the TGFβ signaling pathway (e.g., TGFβ, 5 ng/ml; SB-431542, 10 ng/ml, Tocris). After culture, tissues were processed for RNA isolation or were fixed in 4% paraformaldehyde, incubated in 30% sucrose buffer, embedded in Tissue Tek-O.C.T. compound and stored at −80 °C.
Human tissue specimens. DD tissue was collected during a standard partial fasciectomy procedure. Indications for surgery were contracture(s) of the digit(s) with an inability to put the hand flat on the table (table top test). Only patients with first time occurrence of DD were included in this study. The range of age of patients was between 63 and 88 years old, with 91% being males. Macroscopic identification (with surgical loupe magnification) of a nodule (representing most active disease) was done by the operating surgeon. Only nodules were used in our study. The nodules were defined as being the hard thick parts of the cord, mostly situated in the palm of the hand. This part was taken out of the cord after resection of the entire DD cord. The normal fascia palmaris tissue of the control group was collected during carpal tunnel release procedures. The included control patients did not suffer from DD. The operations were performed under local anesthesia and under tourniquet control. After opening the skin through a longitudinal incision, the fascia was identified and a small piece of fascia was harvested before incising the transverse carpal ligament. The tissue specimen was divided in two equal pieces, one of each was immediately processed for 3D culture and the other one was snap-frozen in liquid nitrogen and stored at −80 °C. Oral consent for removal of the tissue for research purposes was obtained from the patients. Confirmation that the Medical Research Involving Human Subjects Act (WMO) does not apply to the present study was obtained by the local ethics committee (reference number W12_245 # 12.17.0279) since the research was performed on “waste” material.
Antisense oligonucleotides. The AONs used to target ALK5 were developed and recently described in another study, in which in vitro and in vivo efficiency of the different AONs was extensively tested in the context of muscular dystrophies (D.U. Kemaladewi et al., unpublished data). In short, the AONs targeting ALK5 specifically bind to and induce exon skipping of exon 2 of the ALK5 precursor mRNA transcript. Exon 2 encodes for the ligand-binding domain, which is, together with the type 2 receptor, essential in capturing the ligand to initiate signaling. Exclusion of exon 2 will generate a transcript with intact open reading frame, but the resulting protein will lack the ligand binding domain and is therefore functionally impaired. Vivo-morpholino AONs with a morpholino backbone and an octaguanidine moiety to enhance cellular uptake were used in this study since they have been shown to increase exon skipping efficiency in animal models.50 ViM AONs (0.5 and 1 nmol, Genetools, Philomath, OR) were diluted in 1% fetal bovine serum-Dulbecco's modified Eagle's medium or phosphate-buffered saline and were microinjected in the tissue. The sequences (5′-3′) of the ViM AONs are the following: ALK5ViM: GCAGTGGTCCTGATTGCAGCAATAT, ScrViM: CCTCTTACCTCAGTTAC AATTTATA. The 2′-O-methyl ribose AONs with phosphorothioate modifications (2′-O-Me) were obtained by Eurogentec (Belgium). ALK5 2′-O-Me: UGUACAGAGGUGGCAGAAACA, Scr 2′-O-Me: GCAAGAUGCCAGC AGA
RNA isolation, reverse transcription polymerase chain reaction, and quantitative polymerase chain reaction . See Supplementary Materials and Methods for details.
Microscopy and image analysis. See Supplementary Materials and Methods for details.
Immunofluorescence. See Supplementary Materials and Methods for details.
SUPPLEMENTARY MATERIAL Figure S1. Normal fascia palmaris tissue cultures in the 3D ex vivo “clinical trial” system. Figure S2. Time course of ex vivo delivery of ALK5 ViM AON. Figure S3. Quantification of pSMAD2 immunofluorescence by image analysis. Figure S4. Ex vivo delivery of ALK5 AON with ViM and 2'OMe chemical backbones. Materials and Methods
Acknowledgments
This study was supported by Netherlands Organization for Scientific Research (NWO-MW), Netherlands Institute for Regenerative Medicine, Cancer Genomics Centre and Netherlands Centre for Biomedical Genetics. The authors declare no conflict of interest.
Supplementary material
References
- Shih B, Bayat A. Scientific understanding and clinical management of Dupuytren disease. Nat Rev Rheumatol. 2010;6:715–726. doi: 10.1038/nrrheum.2010.180. [DOI] [PubMed] [Google Scholar]
- Hindocha S, Stanley JK, Watson S, Bayat A. Dupuytren's diathesis revisited: Evaluation of prognostic indicators for risk of disease recurrence. J Hand Surg Am. 2006;31:1626–1634. doi: 10.1016/j.jhsa.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Brickley-Parsons D, Glimcher MJ, Smith RJ, Albin R, Adams JP. Biochemical changes in the collagen of the palmar fascia in patients with Dupuytren's disease. J Bone Joint Surg Am. 1981;63:787–797. [PubMed] [Google Scholar]
- Berndt A, Kosmehl H, Katenkamp D, Tauchmann V. Appearance of the myofibroblastic phenotype in Dupuytren's disease is associated with a fibronectin, laminin, collagen type IV and tenascin extracellular matrix. Pathobiology. 1994;62:55–58. doi: 10.1159/000163879. [DOI] [PubMed] [Google Scholar]
- Chen S-J, Yuan W, Mori Y, Levenson A, Trojanowska M, Varga J. Stimulation of type I collagen transcription in human skin fibroblasts by TGF-beta: involvement of Smad. J Invest Dermatol. 1999;112:49–57. doi: 10.1046/j.1523-1747.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGFβeta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochemical Journal. 1987;247:597–604. doi: 10.1042/bj2470597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res. 2000;257:180–189. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Schultz RJ, Haaksma CJ. Extracellular matrix-cytoskeletal connections at the surface of the specialized contractile fibroblast (myofibroblast) in Dupuytren disease. J Bone Joint Surg Am. 1987;69:1400–1407. [PubMed] [Google Scholar]
- Ratajczak-Wielgomas K, Gosk J, Rabczynski J, Augoff K, Podhorska-Okolów M, Gamian A, et al. Expression of MMP-2, TIMP-2, TGF-ß1, and decorin in Dupuytren's contracture. Connect Tissue Res. 2012;53:469–477. doi: 10.3109/03008207.2012.686542. [DOI] [PubMed] [Google Scholar]
- Rehman S, Salway F, Stanley JK, Ollier WE, Day P, Bayat A. Molecular phenotypic descriptors of Dupuytren's disease defined using informatics analysis of the transcriptome. J Hand Surg Am. 2008;33:359–372. doi: 10.1016/j.jhsa.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Kloen P, Jennings CL, Gebhardt MC, Springfield DS, Mankin HJ. Transforming growth factor-beta: possible roles in Dupuytren's contracture. J Hand Surg Am. 1995;20:101–108. doi: 10.1016/S0363-5023(05)80067-X. [DOI] [PubMed] [Google Scholar]
- Dugina V, Fontao L, Chaponnier C, Vasiliev J, Gabbiani G. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J Cell Sci. 2001;114 Pt 18:3285–3296. doi: 10.1242/jcs.114.18.3285. [DOI] [PubMed] [Google Scholar]
- Malmström J, Lindberg H, Lindberg C, Bratt C, Wieslander E, Delander E-L, et al. Transforming growth factor-beta1 specifically induce proteins involved in the myofibroblast contractile apparatus. Molecular & Cellular Proteomics. 2004;3:466–477. doi: 10.1074/mcp.M300108-MCP200. [DOI] [PubMed] [Google Scholar]
- Battegay EJ, Raines EW, Seifert RA, Bowen-Pope DF, Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990;63:515–524. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
- Krause C, Kloen P, Ten Dijke P. Elevated transforming growth factor ß and mitogen-activated protein kinase pathways mediate fibrotic traits of Dupuytren's disease fibroblasts. Fibrogenesis Tissue Repair. 2011;4:14. doi: 10.1186/1755-1536-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayat A, Stanley JK, Watson JS, Ferguson MW, Ollier WE. Genetic susceptibility to Dupuytren's disease: transforming growth factor beta receptor (TGFβetaR) gene polymorphisms and Dupuytren's disease. Br J Plast Surg. 2003;56:328–333. doi: 10.1016/s0007-1226(03)00176-0. [DOI] [PubMed] [Google Scholar]
- Tse R, Howard J, Wu Y, Gan BS. Enhanced Dupuytren's disease fibroblast populated collagen lattice contraction is independent of endogenous active TGF-beta2. BMC Musculoskelet Disord. 2004;5:41. doi: 10.1186/1471-2474-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verjee LS, Verhoekx JS, Chan JK, Krausgruber T, Nicolaidou V, Izadi D, et al. Unraveling the signaling pathways promoting fibrosis in Dupuytren's disease reveals TNF as a therapeutic target. Proc Natl Acad Sci USA. 2013;110:E928–E937. doi: 10.1073/pnas.1301100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aartsma-Rus A, van Vliet L, Hirschi M, Janson AA, Heemskerk H, de Winter CL, et al. Guidelines for antisense oligonucleotide design and insight into splice-modulating mechanisms. Mol Ther. 2009;17:548–553. doi: 10.1038/mt.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aartsma-Rus A, Bremmer-Bout M, Janson AA, den Dunnen JT, van Ommen GJ, van Deutekom JC. Targeted exon skipping as a potential gene correction therapy for Duchenne muscular dystrophy. Neuromuscul Disord. 2002;12 suppl. 1:S71–S77. doi: 10.1016/s0960-8966(02)00086-x. [DOI] [PubMed] [Google Scholar]
- Goemans NM, Tulinius M, van den Akker JT, Burm BE, Ekhart PF, Heuvelmans N, et al. Systemic administration of PRO051 in Duchenne's muscular dystrophy. N Engl J Med. 2011;364:1513–1522. doi: 10.1056/NEJMoa1011367. [DOI] [PubMed] [Google Scholar]
- Cirak S, Arechavala-Gomeza V, Guglieri M, Feng L, Torelli S, Anthony K, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605. doi: 10.1016/S0140-6736(11)60756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellucci VF, Reiss M. Cloning and genomic organization of the human transforming growth factor-beta type I receptor gene. Genomics. 1997;46:278–283. doi: 10.1006/geno.1997.5023. [DOI] [PubMed] [Google Scholar]
- Guillouzo A, Morel F, Ratanasavanh D, Chesne C, Guguen-Guillouzo C. Long-term culture of functional hepatocytes. Toxicol In Vitro. 1990;4:415–427. doi: 10.1016/0887-2333(90)90092-8. [DOI] [PubMed] [Google Scholar]
- O'Gorman DB, Wu Y, Seney S, Zhu RD, Gan BS. Wnt expression is not correlated with beta-catenin dysregulation in Dupuytren's Disease. J Negat Results Biomed. 2006;5:13. doi: 10.1186/1477-5751-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt J, Traynor R, Sapkota GP. The specificities of small molecule inhibitors of the TGFß and BMP pathways. Cell Signal. 2011;23:1831–1842. doi: 10.1016/j.cellsig.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Gilpin D, Coleman S, Hall S, Houston A, Karrasch J, Jones N. Injectable collagenase Clostridium histolyticum: a new nonsurgical treatment for Dupuytren's disease. J Hand Surg Am. 2010;35:2027–38.e1. doi: 10.1016/j.jhsa.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Witthaut J, Jones G, Skrepnik N, Kushner H, Houston A, Lindau TR. Efficacy and safety of collagenase clostridium histolyticum injection for Dupuytren contracture: short-term results from 2 open-label studies. J Hand Surg Am. 2013;38:2–11. doi: 10.1016/j.jhsa.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Kuhn MA, Payne WG, Kierney PC, Pu LL, Smith PD, Siegler K, et al. Cytokine manipulation of explanted Dupuytren's affected human palmar fascia. Int J Surg Investig. 2001;2:443–456. [PubMed] [Google Scholar]
- Olinga P, Groen K, Hof IH, De Kanter R, Koster HJ, Leeman WR, et al. Comparison of five incubation systems for rat liver slices using functional and viability parameters. J Pharmacol Toxicol Methods. 1997;38:59–69. doi: 10.1016/s1056-8719(97)00060-9. [DOI] [PubMed] [Google Scholar]
- van de Bovenkamp M, Groothuis GMM, Draaisma AL, Merema MT, Bezuijen JI, van Gils MJ, et al. Precision-cut liver slices as a new model to study toxicity-induced hepatic stellate cell activation in a physiologic milieu. Toxicological Sciences. 2005;85:632–638. doi: 10.1093/toxsci/kfi127. [DOI] [PubMed] [Google Scholar]
- de Graaf IA, Olinga P, de Jager MH, Merema MT, de Kanter R, van de Kerkhof EG, et al. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat Protoc. 2010;5:1540–1551. doi: 10.1038/nprot.2010.111. [DOI] [PubMed] [Google Scholar]
- van de Bovenkamp M, Groothuis GMM, Meijer DKF, Olinga P. Liver slices as a model to study fibrogenesis and test the effects of anti-fibrotic drugs on fibrogenic cells in human liver. Toxicology in Vitro. 2008;22:771–778. doi: 10.1016/j.tiv.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Fisher R, Ulreich JB, Nazakato PZ, Brendel K. Histological and biochemical evaluation of precision-cut liver slices. Toxicology Mechanisms and Methods. 2001;11:59–79. [Google Scholar]
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- Gottrup F, Agren MS, Karlsmark T. Models for use in wound healing research: a survey focusing on in vitro and in vivo adult soft tissue. Wound Repair Regen. 2000;8:83–96. doi: 10.1046/j.1524-475x.2000.00083.x. [DOI] [PubMed] [Google Scholar]
- Bisson MA, McGrouther DA, Mudera V, Grobbelaar AO. The different characteristics of Dupuytren's disease fibroblasts derived from either nodule or cord: expression of α-smooth muscle actin and the response to stimulation by TGF-β1. The Journal of Hand Surgery: British & European Volume. 2003;28:351–356. doi: 10.1016/s0266-7681(03)00135-9. [DOI] [PubMed] [Google Scholar]
- Townley WA, Baker R, Sheppard N, Grobbelaar AO. Dupuytren's contracture unfolded. BMJ. 2006;332:397–400. doi: 10.1136/bmj.332.7538.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Mudera V. Feedback inhibition of high TGF-β1 concentrations on myofibroblast induction and contraction by Dupuytren's fibroblasts. The Journal of Hand Surgery: British & European Volume. 2006;31:473–483. doi: 10.1016/j.jhsb.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Bayat A, Watson JS, Stanley JK, Ferguson MW, Ollier WE. Genetic susceptibility to dupuytren disease: association of Zf9 transcription factor gene. Plast Reconstr Surg. 2003;111:2133–2139. doi: 10.1097/01.PRS.0000060531.98180.32. [DOI] [PubMed] [Google Scholar]
- Satish L, Gallo PH, Baratz ME, Johnson S, Kathju S. Reversal of TGF-ß1 stimulation of a-smooth muscle actin and extracellular matrix components by cyclic AMP in Dupuytren's-derived fibroblasts. BMC Musculoskelet Disord. 2011;12:113. doi: 10.1186/1471-2474-12-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp J, Seyhan H, Müller B, Lanczak J, Pausch E, Gressner AM, et al. N-acetyl-L-cysteine abrogates fibrogenic properties of fibroblasts isolated from Dupuytren's disease by blunting TGF-beta signalling. J Cell Mol Med. 2006;10:157–165. doi: 10.1111/j.1582-4934.2006.tb00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn MA, Wang X, Payne WG, Ko F, Robson MC. Tamoxifen decreases fibroblast function and downregulates TGF(beta2) in dupuytren's affected palmar fascia. J Surg Res. 2002;103:146–152. doi: 10.1006/jsre.2001.6350. [DOI] [PubMed] [Google Scholar]
- Sorrentino A, Thakur N, Grimsby S, Marcusson A, von Bulow V, Schuster N, et al. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10:1199–1207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- Kim SI, Kwak JH, Na H-J, Kim JK, Ding Y, Choi ME. Transforming growth factor-β (TGF-β1) activates TAK1 via TAB1-mediated autophosphorylation, independent of TGF-β receptor kinase activity in mesangial Cells. Journal of Biological Chemistry. 2009;284:22285–22296. doi: 10.1074/jbc.M109.007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcos PA, Li Y, Jiang S. Vivo-Morpholinos: a non-peptide transporter delivers Morpholinos into a wide array of mouse tissues. BioTechniques. 2008;45:613–4, 616, 618 passim. doi: 10.2144/000113005. [DOI] [PubMed] [Google Scholar]
- Saleh AF, Arzumanov AA, Gait MJ. Overview of alternative oligonucleotide chemistries for exon skipping. Methods Mol Biol. 2012;867:365–378. doi: 10.1007/978-1-61779-767-5_23. [DOI] [PubMed] [Google Scholar]
- Stein EA, Dufour R, Gagne C, Gaudet D, East C, Donovan JM, et al. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation. 2012;126:2283–2292. doi: 10.1161/CIRCULATIONAHA.112.104125. [DOI] [PubMed] [Google Scholar]
- Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, et al. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol. 2009;20:931–941. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Li Y, Morcos PA, Doran TJ, Lu P, Lu QL. Octa-guanidine morpholino restores dystrophin expression in cardiac and skeletal muscles and ameliorates pathology in dystrophic mdx mice. Mol Ther. 2009;17:864–871. doi: 10.1038/mt.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.