Abstract
Purpose
Measuring the effect of cancer interventions must take into account rising cancer incidence now that people live longer because of declines in mortality from cardiovascular disease (CVD). Cancer mortality rates in the population do not accomplish this objective. We sought a measure that would reveal the effects of changing mortality rates from other diseases.
Methods
We obtained annual breast, colorectal, lung, and prostate cancer mortality rates from the Surveillance, Epidemiology, and End Results registries; we obtained noncancer mortality rates from national death certificates, 1975 to 2005. We used life-table methods to calculate the burden of cancer mortality as the average person-years of life lost (PYLL) as a result of cancer (cancer-specific PYLL) and quantify individual—and perhaps offsetting—contributions of the two factors that affect cancer-specific PYLL: mortality rates as a result of cancer and other-cause mortality.
Results
Falling cancer mortality rates reduced the burden of mortality from leading cancers, but increasing cancer incidence as a result of decreasing other-cause mortality rates partially offset this progress. Between 1985 and 1989 and between 2000 and 2004, the burden of lung cancer in males declined by 0.1 year of life lost. This decline reflects the sum of two effects: decreasing lung cancer mortality rates that reduced the average burden of lung cancer mortality by 0.33 years of life lost and declining other-cause mortality rates that raised it by 0.23 years. Other common cancers showed similar patterns.
Conclusion
By using a measure that accounts for increased cancer incidence as a result of improvements in CVD mortality, we find that prior assessments have underestimated the impact of cancer interventions.
INTRODUCTION
The 1971 National Cancer Act led to unprecedented growth in cancer research in the United States1; yet cancer mortality rates scarcely changed while mortality rates from noncancer diseases steadily declined. Between 1970 and 2008, mortality rates from heart disease, cerebrovascular disease, and accidents declined 62%, 73%, and 38%, respectively. In the same period, cancer mortality rates declined just 12%, mostly since 1990,2,3 which led scholars to question the return on investments in cancer prevention, screening, and treatment.4–12 Uncertain returns also prompted the National Cancer Institute (NCI) to convene an extramural committee to evaluate population-level measures of progress against the burden of cancer mortality.13 The Extramural Committee identified the person-years of life lost as a result of cancer (cancer PYLL) as a useful measure of the burden of cancer mortality because it takes into account both changes in cancer mortality rates and changes in other-cause mortality rates.
To date, the leading population-level measures of progress against cancer mortality have been cancer survival time and mortality rates. These measures are ideally suited to assess the direct effect of prevention, screening, and treatment on the cancer itself, but they—by construction—neglect changes in other-cause mortality rates.13–15 The NCI Extramural Committee recommended cancer PYLL averaged across the US adult population to measure cancer burden.13 Conceptually, cancer PYLL equals the gain in life expectancy in the absence of deaths from cancer. Therefore, it directly measures the burden by assessing how many years of life are lost to cancer at the population level.16 However, cancer PYLL has not been well suited for assessing progress over time because changes in cancer PYLL are confounded by changes in noncancer mortality rates, a limitation raised by the NCI Extramural Committee.13 Thus, improvements in cancer PYLL might be offset by increasing incidence of cancer because of decreasing mortality rates from other causes, which would increase life expectancy and allow more time for cancer to develop and be diagnosed.
In this study, we use an established demographic method17 that overcomes the previous limitation in PYLL identified by the NCI Extramural Committee13 and enables us to quantify individual, and perhaps offsetting, contributions of the two factors that affect cancer-specific PYLL: mortality rates as a result of cancer and other-cause mortality. By measuring direct contributions of cancer mortality rates on the burden of cancer mortality, we assess the progress of cancer mortality and changes in its burden since 1985 and examine the pace of progress by cancer type, race, and sex.
METHODS
Patient Data
We obtained breast, colon and rectum (colorectal cancer [CRC]), lung, and prostate cancer incidence and mortality data from the NCI's Surveillance, Epidemiology, and End Results (SEER) 9 registry database. SEER 9 consists of tumor registries in Atlanta, GA; Connecticut; Detroit, MI; Hawaii; Iowa; New Mexico; San Francisco-Oakland, CA; Seattle-Puget Sound, WA; and Utah. The SEER 9 registries, which cover approximately 10% of the US population, form the largest, most representative, and longest running national cancer incidence database. SEER captures virtually all of the cancers occurring in the geographic areas covered by the SEER registries; a person's entry into the registries begins with their diagnosis and ends, if relevant, with their death. All of the SEER sites use the same version of the International Classification of Diseases to assign diagnostic codes. Therefore, users of the SEER database can perform accurate time trend analyses of cancer mortality rates.10,18 We analyzed 1,605,372 cancer cases diagnosed between 1975 and 2005, included only the first matching record for each person, and excluded deaths from cancers identified only by autopsy or death certificate. SEER classifies cancer as the cause of death on the basis of the death certificate, the identity of a primary tumor, and relevant comorbidities. We used one further requirement: the cancer death must have occurred within 10 years of its diagnosis.19,20 For example, cancer deaths occurring in 1985 must have been diagnosed no earlier than 1975. By allowing this 10-year time window between diagnosis and death, we were able to calculate incidence-based mortality rates beginning in 1985 for 1,198,806 incident cancer cases. An incidence-based mortality rate for a specific cancer equals the number of cancer deaths divided by the total number of people residing in the geographic areas covered by the SEER registries. We calculated these mortality rates by cancer type, age group, sex, and calendar year.
To obtain death rates from other causes, we used 1985 to 2005 data from the National Center for Health Statistics (NCHS) Multiple-Cause-of-Death Detail Files. We estimated other-cause mortality rates as the difference between NCHS all-cause mortality rates and SEER incidence-based cancer mortality rates.
Analytic Methods
We assessed progress in reducing the burden of cancer mortality through changes in the PYLL as a result of cancer.17 Cancer PYLL is estimated as the difference in life expectancies calculated from two life-tables: life-table 1 was computed with only other-cause mortality rates and life-table 2 was computed with both cancer and other-cause mortality rates.16,21,22 A life-table estimates a population's life expectancy on the basis of its mortality rates and accounts for the age distribution of the population by transforming mortality rates into probabilities of survival.16,23–25 By denominating cancer PYLL on the US adult population age ≥ 40 years, the measure can be evaluated on the same scale as life expectancy. Thus, we are able to assess progress in reducing the burden of adult cancer mortality.
We overcome the previous limitations in interpreting time trends in PYLL (an issue raised by the NCI Extramural Committee) by quantifying individual contributions of the two factors that affect cancer PYLL: cancer and other-cause mortality rates.17 We schematically represent our approach in Figure 1 and fully describe it in the Data Supplement. We performed this analysis for specific cancers in people age 40 to 84 years over consecutive 5-year periods beginning with 1985 to 1989 through 2000 to 2004.
Fig 1.
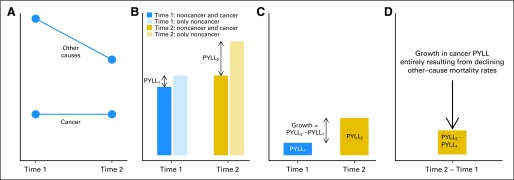
Changes in the average person-years of life lost (PYLL) depend on changes in mortality rates from both cancer and other causes. (A) Mortality rates for cancer and other causes at two time points. (B) Life expectancy calculated from cancer and other-cause mortality rates (blue and gold) and life expectancy calculated from only other-cause mortality rates for time 1 (light blue) and time 2 (light gold). (C) Cancer PYLL equals the difference in life expectancy calculated from only other-cause mortality rates and life expectancy calculated from cancer plus other-cause mortality rates. (D) Change in cancer PYLL equals the difference between cancer PYLL at time 2 and cancer PYLL at time 1.
Conceptually, cancer PYLL depends on the competing risks of cancer and noncancer mortality. For example, suppose cancer mortality rates remained constant between times 1 and 2 but noncancer mortality rates declined (Fig 1A). At time 1, the life expectancy based only on noncancer mortality rates is higher than the life expectancy based on both noncancer and cancer mortality rates (Fig 1B). The difference between these life expectancies represents the PYLL as a result of cancer averaged across the population (cancer PYLL). At time 2, cancer PYLL is even greater than at time 1 because noncancer mortality rates declined over time (Fig 1C). Cancer mortality did not contribute to the change in cancer PYLL because cancer mortality rates themselves had not changed. Therefore, decreases in noncancer mortality rates confound the interpretation of changes in cancer PYLL (Fig 1D). In this example, all of the increase in cancer PYLL is a result of decreasing noncancer mortality rates. We do not report any sampling uncertainty in cancer PYLL because our calculations use registry and vital statistics data that fully capture the mortality experience of defined populations.25 The Harvard University institutional review board approved the study.
RESULTS
Average PYLL
To demonstrate the principal results of our investigation, we used the leading cause of cancer deaths—lung cancer—and consider changes in the burden of male lung cancer (measured by PYLL) as a result of changes in lung cancer mortality rates (Fig 2). In 1985, the life expectancy by using life-tables based only on other-cause mortality rates was 0.87 years higher than the life expectancy using life-tables based on both lung cancer and other-cause mortality rates. By the definition of PYLL, the lung cancer PYLL1985 is 0.87 years (Fig 2A). By 1990, lung cancer PYLL grew to 0.90 years, an increase of 0.03 years. As shown in the first set of bars in Fig 2B, this growth in PYLL (gold bar) resulted from the combined effect of decreasing other-cause mortality rates (blue bar), which raised lung cancer PYLL by 0.07 years, and decreasing lung cancer mortality rates (gray bar), which lowered PYLL 0.04 years. The pattern of gold bars over time (Fig 2A) shows that the burden of lung cancer mortality shrank since 1990. This shrinkage was primarily the result of decreasing lung cancer mortality rates (Fig 2B, gray bars) that consistently reduced the burden of lung cancer mortality, an effect that was partially offset by decreasing other-cause mortality rates (blue bars, Fig 2B). Decreasing lung cancer mortality rates tripled their contribution to reducing the burden of lung cancer mortality from 0.04 years in 1985 to 1989 to 0.12 years in 2000 to 2004. Not all of this progress was realized at the population level because other-cause mortality rates also decreased, and the resulting increase in life expectancy and consequent change in lung cancer incidence partially offset this progress.
Fig 2.
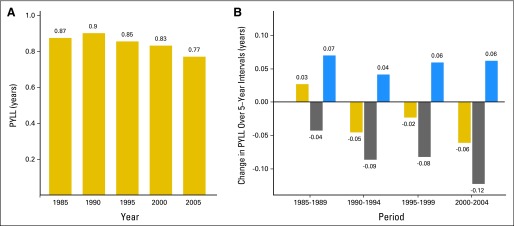
Male lung cancer average person-years of life lost (PYLL) by year, and its changes and decomposition over 5-year intervals showing large reductions in male lung cancer burden as a result of declines in lung cancer mortality rates since 1985. (A) Male lung cancer PYLL by year. (B) Decomposition of male lung cancer PYLL over 5-year intervals. Gold bars indicate the change in PYLL, gray bars indicate the change in PYLL as a result of changes in lung cancer mortality rates, and blue bars indicate the change in PYLL as a result of changes in other-cause mortality rates. We do not report any sampling uncertainty in PYLL or in the decomposition of PYLL because our calculations use registry and vital statistics data that fully capture the mortality experience of defined populations. Note that PYLL, the average years of life lost per US adult, equals the gain in life expectancy in the absence of deaths resulting from cancer.
Time Trends and Decomposition of Average PYLL by Sex, Race, and Cancer Site
Decreasing cancer mortality rates reduced the burden of specific cancers, although the timing and magnitude has varied among cancers and sex (Data Supplement). For females, the burden of lung cancer mortality grew from 1985 to 1989 and from 1995 to 1999, but in contrast to the decline in males, it remained nearly constant thereafter. Although the burden of lung cancer mortality appeared constant, decreasing lung cancer mortality rates in women actually reduced it, but this reduction was offset by nearly equal growth in cancer PYLL from declining other-cause mortality rates. For example, between 2000 and 2004, decreasing lung cancer mortality rates reduced the burden of lung cancer mortality in women by 0.04 years and declining other-cause mortality rates raised it by 0.03 years, leading to an overall 0.01 years of reduction in the burden of lung cancer mortality. Other cancers showed similar patterns. During 1985 to 1989, decreasing CRC mortality rates for both males and females reduced the burden of CRC mortality, although this reduction was more consistent for females than males. Decreases in breast cancer mortality rates consistently reduced the burden of breast cancer mortality from 1985 to 1989 with the greatest reduction occurring from 1995 to 1999. Finally, most of the reduction in the burden of prostate cancer mortality from 1990 to 1994 was the result of decreases in prostate cancer mortality rates, with relatively little offsetting contribution from lower other-cause mortality rates.
Last, we examined patterns in the burden of cancers by sex and race and found that although the burdens of lung and prostate cancer mortality shrank more for black than for white males, the burdens of breast cancer and CRC mortality shrank more consistently for white than for black females (Data Supplement). For example, between 2000 and 2004, decreasing lung cancer mortality rates reduced the burden of lung cancer mortality by 0.16 years for black males and 0.12 years for white males but by only 0.06 years for black females and 0.05 years for white females.
DISCUSSION
Our methods provide a more accurate means of assessing progress against the population-level burden of cancer mortality as measured in average PYLL as a result of cancer. We show that accurately measuring progress depends on changes in mortality rates of both cancer and other diseases. We estimate how the years of life lost from cancer are directly affected by cancer mortality itself and indirectly affected by increased cancer incidence because of greater longevity as a result of improvements in the prevention, detection, and treatment of other diseases.
Our study realizes the promise of the conceptual advances made by the 1990 NCI Extramural Committee. The committee noted an urgent need for research into how changes in other causes of death affect cancer statistics.13 The committee found PYLL to be an appealing measure of progress because it incorporates mortality rates from both cancer and other diseases. However, the committee identified an important drawback of using PYLL to assess progress against cancer over time—PYLL will worsen when other-cause mortality rates decline, even if cancer mortality rates remain constant. To the best of our knowledge, no subsequent work has addressed this drawback of PYLL. For the first time, our approach overcomes this problem by quantifying the contribution of changes in mortality rates from cancer and other diseases to changes in PYLL from cancer. We are now able to conclude that seemingly stagnant progress against many leading cancers (eg, female lung cancer between 1995 and 2005) actually resulted from offsetting contributions of decreasing cancer mortality rates and decreasing other-cause mortality rates.
Our study introduces, for the first time, an approach to assessing both the burden of cancer and progress over time. It has been possible to measure each of these characteristics individually but not both at the same time. For example, the prevalence of cancer and years of life lost as a result of cancer are, in principle, ideal measures of the burden of cancer mortality because they explicitly consider competing mortality from other causes of death. Relative survival (ie, the ratio of life expectancy for patients with cancer to life expectancy for cancer-free individuals) and standardized mortality rates (the weighted sum of age-specific cancer mortality rates, in which the weights equal the proportion of the population in each age group) are, in principle, ideal measures of progress against cancer mortality because they focus exclusively on cancer.10 However, none of these measures assess both the burden of cancer and progress of this burden over time; changes in the burden result from changes in cancer mortality rates but are confounded by changes in other-cause mortality rates. In contrast, we used cancer PYLL as a measure of burden and assessed progress against the burden of cancer mortality over time by quantifying how much of the change in PYLL is a result of mortality rates from cancer and from other causes. Our approach accounts for the increase in cancer prevalence as a result of longer lifetime in which to develop cancer. In addition, by using life-table methods, our measure accounts for changes in the proportion of the population by age over time and therefore allows meaningful comparisons across time and population subgroups (eg, race/ethnicity).
Our results demonstrate progress against the burden of cancer mortality, but we show that conventional measures may underestimate this progress because they do not measure the offsetting effects that result from larger improvements in the mortality of noncancer diseases.26 By quantifying the contribution of cancer and noncancer diseases to the burden of cancer mortality, we reveal more accurately the aggregate contribution of cancer prevention, screening, and treatment on progress against cancer.
Our study has some potential limitations, which may affect its internal and external validity. First, we required that cancer death must have occurred within 10 years of diagnosis when calculating incidence-based mortality rates. This 10-year window ensures that incidence-based cancer mortality rates are within 10% of cancer death rates estimated from national death certificate data.19 As screening became more widespread over time, a larger number of cancers were diagnosed at the earliest stages and may have resulted in a higher proportion of indolent cancers that lead to an underestimation of cancer mortality rates. To address this potential problem, we set a 10-year window to reduce the effect of indolent cancer on cancer mortality rates. For our sensitivity analysis, we varied the time interval by 2.5-year increments between 5 and 15 years and reached nearly identical substantive conclusions regarding the timing and magnitude of progress against the burden of cancer mortality (Data Supplement). Second, the SEER registries do not capture the entire US population. Our results may not be generalizable to the national population to the extent that the SEER registries fail to represent more general cancer mortality trends. For example, screening rates for breast and prostate cancer and CRC may be higher in the SEER registries than in the rest of the United States, which may result in a higher proportion of indolent cancers and an underestimation of cancer mortality rates. To address this potential problem, we conducted additional analysis by using only national-level mortality data, and we reached similar conclusions (Data Supplement). Although national-level mortality data capture the entire US population, they rely on death certificates. We were not able to ascertain the date of cancer diagnosis from death certificates for calculating incidence-based mortality rates as we could by using SEER registry data. Third, our method assumes causes of death to be mutually independent (ie, cancer death rates are not correlated with noncancer death rates) when estimating the contribution of each cancer to the change in the burden of cancer mortality.17 This assumption may be reasonable in younger patients with cancer who have fewer comorbid conditions but may be more tenuous in older patients when the prevalence of serious advanced chronic disease is greater and can shorten cancer survival. We address this assumption by restricting the age range in our analysis to people age 40 to 84 years for whom the prevalence of multiple comorbidities is lower than among people age 85 years or older.27 Other methods to assess progress against cancer mortality relax the mutual independence assumption among causes of death but, in doing so, invoke untestable assumptions about both the competition among multiple causes of death28 and the interrelationship of comorbid diseases and disease progression.29,30
In conclusion, as mortality from cardiovascular disease and other major diseases continues to decline, the population-level burden of cancer mortality may grow because life expectancy will increase, leading to more years of life for cancer to develop and be diagnosed. Our framework suggests that the average age of cancer diagnosis increases as the exposure to cancer lengthens; this pattern has been empirically observed for many leading cancers.31 Given the decline in noncancer mortality rates, assessing the direct contribution of cancer mortality rates on the burden of cancer mortality requires new methodologies that distinguish the effects of cancer care from the effects of medical care that reduce mortality from other diseases. We show that the average PYLL resulting from cancer is a useful metric of the burden of cancer mortality because it jointly considers changes in cancer and other-cause mortality rates and is denominated on the population as a whole. By decomposing changes in the average PYLL as a result of cancer over time, we are then able to assess the contribution of cancer-specific mortality rates on changes in the burden of cancer mortality, adjusting for progress in reducing other causes of death. In doing so, we find sustained progress since the years from 1985 to 1989 in reducing the population-level burden of cancer mortality.
Supplementary Material
Acknowledgment
We thank David Asch, Valerie Lewis, Analia Olgiati, Jonathan Skinner, Rania Salem, and Anna Tosteson and three anonymous reviewers for helpful comments and suggestions.
Footnotes
Supported by Grant No. RC2CA148259 from the National Cancer Institute and Grant No. T32AG0037 from the National Institute of Aging and by the Harvard Center for Population and Development Studies.
Presented in part at the 37th Annual Meeting of the American Society of Preventive Oncology, Memphis, TN, March 9-12, 2013.
The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Samir Soneji, Hiram Beltrán-Sánchez
Collection and assembly of data: Samir Soneji, Hiram Beltrán-Sánchez
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Kiberstis P, Marshall E. Cancer crusade at 40: Celebrating an anniversary—Introduction. Science. 2011;331:1539. doi: 10.1126/science.331.6024.1539-a. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Ward E, Hao Y, et al. Trends in the leading causes of death in the United States, 1970-2002. JAMA. 2005;294:1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Census Bureau. The 2012 Statistical Abstract of the United States: Table 117—Age-adjusted death rates by major causes. www.census.gov/compendia/statab/cats/births_deaths_marriages_divorces.html.
- 4.Bailar JC, 3rd, Smith EM. Progress against cancer? N Engl J Med. 1986;314:1226–1232. doi: 10.1056/NEJM198605083141905. [DOI] [PubMed] [Google Scholar]
- 5.Chelimsky E. Washington, DC: US General Accounting Office; 1987. Cancer patient survival: What progress has been made? [Google Scholar]
- 6.Bailar JC, 3rd, Gornik HL. Cancer undefeated. N Engl J Med. 1997;336:1569–1574. doi: 10.1056/NEJM199705293362206. [DOI] [PubMed] [Google Scholar]
- 7.Freeman HP, Rueben SH. The National Cancer Program: Assessing the Past, Charting the Future. President's Cancer Panel. 1999 [Google Scholar]
- 8.Haynes MA, Smedley BD, editors. The Unequal Burden of Cancer: An Assessment of NIH Research and Programs for Ethnic Minorities and the Medically Underserved. Washington, DC: The National Academies Press; 1999. Institute of Medicine Committee on Cancer Research Among Minorities and the Medically Underserved. [PubMed] [Google Scholar]
- 9.Cutler DM. Are we finally winning the war on cancer? J Econ Perspect. 2008;22:3–26. doi: 10.1257/jep.22.4.3. [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Ward E, Thun M. Declining death rates reflect progress against cancer. PLoS One. 2010;5:e9584. doi: 10.1371/journal.pone.0009584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gapstur SM, Thun MJ. Progress in the war on cancer. JAMA. 2010;303:1084–1085. doi: 10.1001/jama.2010.284. [DOI] [PubMed] [Google Scholar]
- 12.Tiwari AK, Roy HK. Progress against cancer (1971-2011): How far have we come? J Intern Med. 2012;271:392–399. doi: 10.1111/j.1365-2796.2011.02462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Measurement of progress against cancer: Extramural Committee to Assess Measures of Progress Against Cancer. J Natl Cancer Inst. 1990;82:825–835. [No authors listed] [PubMed] [Google Scholar]
- 14.Welch HG, Schwartz LM, Woloshin S. Are increasing 5-year survival rates evidence of success against cancer? JAMA. 2000;283:2975–2978. doi: 10.1001/jama.283.22.2975. [DOI] [PubMed] [Google Scholar]
- 15.Thorpe KE, Howard DH, Galactionova K. Differences in disease prevalence as a source of the U.S.-European health care spending gap. Health Aff (Millwood) 2007;26:w678–w686. doi: 10.1377/hlthaff.26.6.w678. [DOI] [PubMed] [Google Scholar]
- 16.Preston SH, Heuveline P, Guillot M. Demography: Measuring and Modeling Population Processes. Oxford, United Kingdom: Blackwell Publishers; 2001. [Google Scholar]
- 17.Beltrán-Sánchez H, Preston SH, Canudas-Romo V. An integrated approach to cause-of-death analysis: Cause-deleted life tables and decompositions of life expectancy. Demogr Res. 2008;19:1323. doi: 10.4054/DemRes.2008.19.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–736. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu KC, Miller BA, Feuer EJ, et al. A method for partitioning cancer mortality trends by factors associated with diagnosis: An application to female breast cancer. J Clin Epidemiol. 1994;47:1451–1461. doi: 10.1016/0895-4356(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 20.Chu KC, Tarone RE, Freeman HP. Trends in prostate cancer mortality among black men and white men in the United States. Cancer. 2003;97:1507–1516. doi: 10.1002/cncr.11212. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute. Bethesda, MD: National Cancer Institute, National Institutes of Health, Department of Health and Human Services; 2012. Aug, Cancer Trends Progress Report: 2011/2012 Update. http://progressreport.cancer.gov. [Google Scholar]
- 22.Liu PH, Wang JD, Keating NL. Expected years of life lost for six potentially preventable cancers in the United States. Prev Med. 2013;56:309–313. doi: 10.1016/j.ypmed.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Anderson RN. Method for Constructing Complete Annual U.S. Life Tables. Hyattsville, MD: National Center for Health Statistics; 1999. [PubMed] [Google Scholar]
- 24.Arias E. United States Life Tables, 2008. Hyattsville, MD: National Center for Health Statistics; 2012. [PubMed] [Google Scholar]
- 25.King G, Zeng L. Explaining rare events in international relations. Int Organ. 2001;55:693–715. [Google Scholar]
- 26.Ergin A, Muntner P, Sherwin R, et al. Secular trends in cardiovascular disease mortality, incidence, and case fatality rates in adults in the United States. Am J Med. 2004;117:219–227. doi: 10.1016/j.amjmed.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Piccirillo JF, Vlahiotis A, Barrett LB, et al. The changing prevalence of comorbidity across the age spectrum. Crit Rev Oncol Hematol. 2008;67:124–132. doi: 10.1016/j.critrevonc.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honoré BE, Lleras-Muney A. Bounds in competing risks models and the war on cancer. Econometrica. 2006;74:1675–1698. [Google Scholar]
- 29.Tsiatis A. A nonidentifiability aspect of the problem of competing risks. Proc Natl Acad Sci U S A. 1975;72:20–22. doi: 10.1073/pnas.72.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yashin AI, Manton KG, Stallard E. Dependent competing risks: A stochastic process model. J Math Biol. 1986;24:119–140. doi: 10.1007/BF00275995. [DOI] [PubMed] [Google Scholar]
- 31.Hayat MJ, Howlader N, Reichman ME, et al. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12:20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


