Abstract
Smoking is approximately three times more prevalent in HIV-1–positive than HIV-negative individuals in the United States. Nicotine, which is the major constituent of tobacco, is rapidly metabolized mainly by cytochrome P450 (CYP2A6) to many metabolites. In this study, we developed a simple, fast, and sensitive electrospray ionization liquid chromatography–tandem mass spectrometry method using a strong cation solid phase extraction, and determined the concentration of nicotine and its four major metabolites (cotinine, nornicotine, norcotinine, and trans-3′-hydroxycotinine) in the plasma of HIV-1–positive and HIV-negative smokers. The multiple reaction monitoring transitions for nicotine, cotinine, trans-3′-hydroxycotinine, nornicotine, norcotinine, nicotine-d4, and cotinine-d3 were selected at mass-to-charge ratios of 163.3/117.1, 177.5/80.3, 193.2/80.1, 149.5/132.3, 163.4/80.3, 167.3/121.4, and 180.3/101.2, respectively. The lower limit of quantitation for nicotine and its metabolites was 0.53 ng/ml, which is relatively more sensitive than those previously reported. The concentration of nicotine was detected 5-fold lower in HIV-1–positive smokers (7.17 ± 3.8 ng/ml) than that observed in HIV-negative smokers (33.29 ± 15.4 ng/ml), whereas the concentration of the metabolite nornicotine was 3-fold higher in HIV-1–positive smokers (6.8 ± 2.9 ng/ml) than in HIV-negative smokers (2.3 ± 1.2 ng/ml). Although it was statistically nonsignificant, the concentration of the metabolite cotinine was also higher in HIV-1–positive smokers (85.6 ± 60.5 ng/ml) than in HIV-negative smokers (74.9 ± 40.5 ng/ml). In conclusion, a decrease in the concentration of nicotine and an increase in the concentration of its metabolites in HIV-1–positive smokers compared with HIV-negative smokers support the hypothesis that nicotine metabolism is enhanced in HIV-1–positive smokers compared with HIV-negative smokers.
Introduction
Tobacco smoking in the United States is approximately three times more prevalent in individuals infected with HIV-1 (approximately 60%) than in uninfected individuals (approximately 20%) (Burkhalter et al., 2005). There is increasing evidence that smoking/nicotine increases HIV-1 replication in vitro, particularly in alveolar macrophages, microglia, and T cells (Abbud et al., 1995; Rock et al., 2008; Zhao et al., 2010). Since nicotine exposure has been shown to disrupt the blood–brain barrier (Manda et al., 2010), HIV-1–infected monocytes/macrophages can infiltrate the brain and infect microglia, astrocytes, and perivascular macrophages (Kedzierska and Crowe, 2002; Montaner et al., 2006). In addition, smoking has been shown to decrease the response to antiretroviral therapy that enhances the risk of viral replication (Feldman et al., 2006; Wojna et al., 2007).
Nicotine is the main bioactive natural alkaloid present in tobacco, which is rapidly absorbed into the peripheral blood and penetrates the brain. In addition, nicotine is metabolized through many complex pathways and forms several metabolites by phase I (N-oxidation, C-oxidation, hydroxylation, and N-demethylation) and phase II (glucuronic acid) (Benowitz and Jacob, 1994) metabolic reactions. Nicotine is extensively metabolized by liver CYP2A6 using C-oxidation to major metabolite cotinine and other minor metabolites such as cotinine-N-oxide and nornicotine. Cotinine is further hydroxylated by CYP2A6 to trans-3′-hydroxycotinine and forms norcotinine upon N-methylation, as well as other minor metabolites (Benowitz, 2009). CYP2A6-mediated metabolism of nicotine produces metabolites (some of which are toxic) that are known to cause oxidative stress leading to liver damage and pulmonary and pancreatic cancers (Benowitz, 2008, 2009; Kadlubar et al., 2009). In addition, nicotine is known to be toxic to other cells, including brain (Bhagwat et al., 1998). We recently showed the expression of CYP2A6 in astrocytes and monocytes, as well as the role of CYP2A6 in nicotine metabolism to cotinine that induces the formation of reactive oxygen species (ROS) (Jin et al., 2011, 2012a,b; Ande et al., 2012). Furthermore, we have shown that the ROS generated through ethanol metabolism by CYP2E1 induces the expression of CYP2A6 in monocytes (Jin et al., 2012b)
Earlier findings have shown that oxidative stress increases HIV-1 replication in alveolar macrophages from smokers (Boelaert et al., 1996; Israel and Gougerot-Pocidalo, 1997; Aquaro et al., 2007). Since our recent findings show the role of nicotine metabolism by CYP2A6 in ROS production (Ande et al., 2012; Jin et al., 2012a), we sought to determine the plasma levels of nicotine and four of its metabolites that are formed by CYP2A6 in HIV-1–positive smokers. Nicotine and its metabolites have primarily been studied in urine and to some extent in other biologic matrices such as serum, plasma, hair, and nails using immunoassay, high-performance liquid chromatography (HPLC), gas chromatography–mass spectrometry, and liquid chromatography–mass spectrometry with protein precipitation, liquid extraction methods, and solid phase extraction (SPE) methods (Jarvis et al., 2003; Byrd et al., 2005; Shu and Wang, 2013). However, the usefulness of these methods has been limited by factors such as the requirement of high sample volume and the lengthy extraction process, among other impediments. The plasma concentration of nicotine and its metabolites is generally much lower than that in urine (Jarvis et al., 1987). It is therefore necessary to develop a more sensitive analytical technique using a liquid chromatography–tandem mass spectrometry (LC-MS/MS) instrument with a triple quadrupole linear ion trap, which is commonly employed for the determination of low levels of nicotine and its metabolites. A previous study showed the application of the LC-MS/MS method for quantifying only cotinine at a sensitivity of 0.5 ng/ml (Beyer et al., 2007; Bernert et al., 2009). Subsequently, another LC-MS/MS method using SPE was developed that was able to quantitate two analytes, nicotine and cotinine, with a sensitivity of 2 ng/ml (Byrd et al., 2005). Several LC-MS/MS analytical techniques have recently been reported to determine nicotine, cotinine, and trans-3′-hydroxycotinine with a sensitivity of 1 ng/ml; however, in these methods, the sample preparation and extraction were time-consuming and tedious, required high sample volume, and were limited to two to three analytes (Miller et al., 2010; Jacob et al., 2011). Therefore, our objective in this study was to develop a simple, fast, and sensitive electrospray ionization (ESI)–LC-MS/MS technique using a SPE cartridge for the simultaneous determination of nicotine and its four metabolites in plasma. In this communication, we further report the concentrations of nicotine and its metabolites detected in plasma from HIV-1–positive and HIV-negative smokers using the above approach.
Materials and Methods
Chemical and Reagents.
Nicotine, cotinine, trans-3′-hydroxycotinine, nornicotine, norcotinine, nicotine-d4, and cotinine-d3 were purchased from Cerilliant Analytical Reference Standards (Sigma-Aldrich Company, Round Rock, TX). HPLC-grade methanol, acetonitrile, ammonia solution, and formic acid were procured from Fisher Scientific (New Brunswick, NJ). Ultrapure water from a MilliQ-system (Millipore, Molshecin, France) was used in this study. All HPLC-grade chemicals were used without further purification. An Exterra HPLC reverse phase MS C18 column (Waters Corporation, Milford, MA) and strong cation SPE cartridges (Agility DVB; Orochem Technologies, Lombard, IL) were employed for the determination of these analytes.
Solutions and Reference Standards.
Nicotine, cotinine, trans-3′-hydroxycotinine, nornicotine, norcotinine, and the internal standards (ISs) (deuterated nicotine-d4 and cotinine-d3) were dissolved at a concentration of 1 mg/ml in 80% methanol (v/v). To prepare the standard curve, 20 µl of a working solution at different concentrations (12.60 to 5.04, 2.02, 0.76, 0.25, 0.076, 0.027, and 0.013 µg/ml) was spiked into 500 µl plasma from a nonsmoker (blank) to obtain calibration standards of decreasing concentrations (504.0, 201.6, 80.6, 30.6, 10.1, 3.03, 1.06, and 0.53 ng/ml). Similarly, the quality control (QC) samples were independently prepared at four concentrations (504.0, 80.6, 3.03, and 1.06 ng/ml) in plasma from nonsmokers. Stock concentrations were corrected as described in our previous communication (Earla et al., 2012).
System Suitability and Carry-Over Tests.
The system suitability test was done to assess the instrument performance in terms of chromatographic peak resolution, peak retention time, and consistency of the peak area response from day-to-day applications. This was performed by analyzing six replicate injections of 500 ng/ml reference standard compared with the IS. The system suitability test for each analyte was performed by calculating the coefficient of variation (CV) (standard deviation/mean concentration) multiplied by 100. The carry-over test was performed in triplicate by injecting a blank plasma sample extract followed by immediate injection of an extract of the sample from the upper limit of the standard curve (ULOQ) along with an IS.
Tandem Mass Spectrometry Conditions.
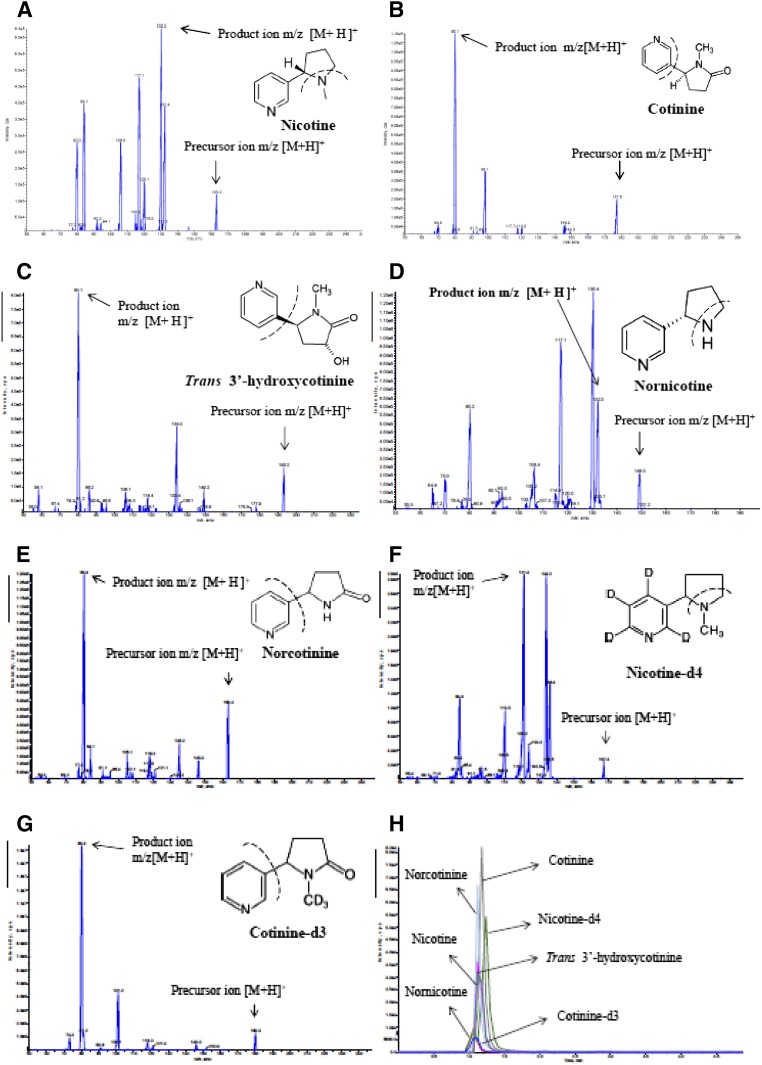
The mass spectrometer (3200 QTRAP LC-MS/MS system; AB Sciex, Foster City, CA) was optimized for detection of nicotine and its metabolites along with IS by using 200 ng/ml. Mass spectrometry data of each compound were first acquired in full-scan mode from the range between 50 and 300 Da to identify their precursor ions. The most suitable proton adduct in the positive mode [M+H]+ precursor ions was determined for nicotine (163.3), cotinine (177.5), trans-3′-hydroxycotinine (193.2), nornicotine (149.5), norcotinine (163.4), nicotine-d4 (167.3), and cotinine-d3 (180.3) (Fig. 1; Supplemental Table 1). These precursor ions were optimized by setting the curtain gas, declustering potential, ion spray voltage, and source gas 1.
Fig. 1.
MS/MS spectra of nicotine (A), cotinine (B), trans-3′-hydroxycotinine (C), nornicotine (D), norcotinine (E), nicotine-d4 (IS) (F), and cotinine-d3 (IS) (G) with proton adducts [M+H]+ in ESI-positive mode. (H) MRM chromatogram of a mixture of reference standard containing nicotine, cotinine, trans-3′-hydroxycotine, nornicotine, norcotinine, nicotine-d4, and cotinine-d3. These are standard chemicals and were made using ChemDraw Ultra (version 6.0.1; CambridgeSoft.com).
The proton adduct mass-to-charge ratio (m/z) [M+H] + precursor ions of nicotine and its metabolites along with IS were selected in positive mode for collision cell quadrupole 2 (MS2). Precursor ions were fragmented by applying collisionally activated dissociation gas and collision energy to obtain their most abundant and stable product ions. The product ions for nicotine (117.1), cotinine (80.3), trans-3′-hydroxycotinine (80.1), nornicotine (132.3), norcotinine (80.3), nicotine-d4 (121.4), and cotinine-d3 (101.2) were optimized by adjusting collision energy, curtain gas, entrance potentials, and source gas 2 (Fig. 1; Supplemental Table 1). The following multiple reaction monitoring (MRM) transitions (m/z) [M+H]+ (Q1→Q3) were selected for quantitative analyses: 163.3→117.1 for nicotine, 177.5→80.3 for cotinine, 193.2→80.1 for trans-3′-hydroxycotinine, 149.5→132.3 for nornicotine, 163.4→80.3 for norcotinine, 167.3→121.4 for nicotine-d4, and 180.3→101.2 for cotinine-d3 (Fig. 1; Supplemental Table 1). A dwell time of 500 ms and a source temperature of 400°C were employed for all of the analyte determinations.
LC-MS/MS Chromatographic Separation.
A LC-MS/MS chromatographic separation was achieved by a reverse phase XTerra MS C18 column (50 × 4.6 mm, i.d, 5µm) using a Ultra-Fast Liquid Chromatography Shimadzu LC-20AD HPLC (Shimadzu Scientific Instruments, Pleasanton, CA). An isocratic mobile phase composed of 55% acetonitrile in water containing 0.05% of formic acid at a flow rate of 0.4 ml/min was used. The samples were reconstituted in a solution of 500 µl acetonitrile/water/formic acid (70:30:0.05). A 15-µl aliquot of each sample was injected into LC-MS/MS for quantitative analysis for 5 minutes. The LC-MS/MS–acquired MRM data were processed with Analyst software (version 1.4.2; AB Sciex).
Sample Preparation and Extraction.
A simple SPE technique was used for sample extraction. Five hundred microliters of plasma from a nonsmoker was aliquoted and 20 µl of 3 µg/ml IS (final concentration of approximately 0.1 µg/ml) was added. The mixture was vortex-mixed for 30 seconds followed by addition of 25 µl aqueous 0.6% formic acid solution, which was again vortex-mixed for 1 minute prior to SPE. The strong cation SPE columns (SCX 30 mg, 1 ml cartridge) were preconditioned with 1 ml methanol and equilibrated with 1 ml 0.6% formic acid in water. The plasma samples were loaded on the SPE cartridge and drained slowly by applying positive pressure at 15 psi with a 48-well plate positive vacuum manifold. The SPE columns were washed with 1 ml 0.6% formic acid followed by 1 ml each of water and methanol. Next, the SPE columns were air-dried under a positive vacuum at 20 psi for 3 minutes, and analytes were eluted with 1 ml 7% ammonium hydroxide in methanol. After elution, the pH of the eluate was neutralized with 100 µl 6% formic acid in methanol and vortex-mixed prior to evaporation using a speed vacuum at 35°C for 60 minutes. Samples were redissolved in 500 µl reconstitution solution.
Specificity and Selectivity.
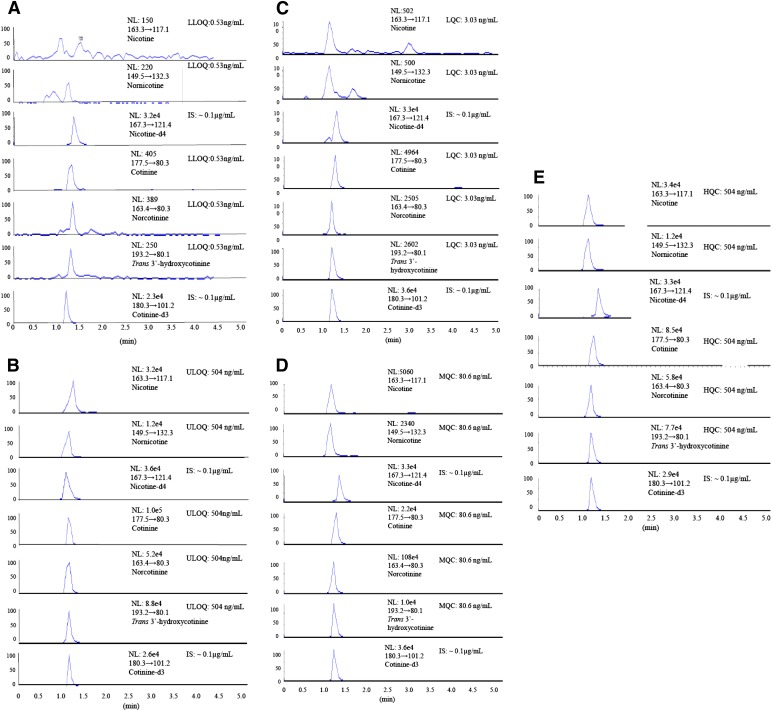
Specificity and selectivity were tested by analyzing six blank plasma samples from HIV-negative nonsmokers. These blank matrices were used for method standardization, which did not show measurable interference at analyte peak of interests for nicotine and its metabolites. The lower limit of quantitation (LLOQ) (0.53 ng/ml) of six samples was processed to assess the blank plasma interference at the analyte peak of interest (Fig. 2A; Supplemental Fig. 1). The percentage of interference determined in the blank was calculated by comparing the mean peak area of LLOQ of the analyte with the peak response obtained from the blank samples. The peak areas of blanks coeluting with the analytes were required to be less than 5 times of the mean peak area at the LLOQ.
Fig. 2.
LC-MS/MS MRM chromatograms of nicotine, its metabolites, and ISs. Extracted LLOQ (0.53 ng/ml) with IS (A), extracted ULOQ (504 ng/ml) with IS (B), extracted LQC (3.03 ng/ml) standard (C), extracted MQC (80.6 ng/ml) (D), and extracted HQC (504 ng/ml) (E) from plasma of nonsmokers.
Precision and Accuracy.
Within-day and between-day precision and accuracy experiments were performed by analyzing eight extracted calibrations and four levels of QC standards. The pooled blank plasma samples from HIV-negative nonsmokers were used to prepare a calibration curve and QC standards. The standard samples were prepared based on the procedure described earlier (Earla et al., 2010). Precision was determined by analyzing six replicates at each of the four levels of QC standards. Accuracy was reported as the percentage difference between the mean concentrations divided by the nominal concentration, multiplied by 100. Accuracy was required to be ±15% of the nominal value of all of the standards, except at the LLOQ level, in which an accuracy of ±20% was accepted according to the Guidance for Industry Bioanalytical Method Validation in US Food and Drug Administration guidelines published in May 2001 (http://www.fda.gov). Precision was calculated using the CV (standard deviation/mean concentration) multiplied by 100. Precision of the method was required to be ≤15% of the nominal concentration except in the LLOQ, in which ≤20% was an accepted CV (Table 1).
TABLE 1.
Intraday and interday precision and accuracy of CC and four levels of QC standards for nicotine, nornicotine, cotinine, norcotinine, and trans-3′-hydroxycotinine in pooled human plasma from HIV-negative nonsmokers
| Analyte | Nominal Concentration | Within-Day: CC (n = 4), QC (n = 6) |
Between-Day: CC (n = 6), QC (n = 6) |
||||
|---|---|---|---|---|---|---|---|
| Calculated Concentration | Precision | Accuracy | Calculated Concentration | Precision | Accuracy | ||
| ng/ml | ng/ml | %CV | % | ng/ml | %CV | % | |
| Nicotine | |||||||
| CC standard-1 | 504.00 | 476.57 | 7.6 | 94.6 | 543.9 | 3.8 | 107.9 |
| CC standard-2 | 201.60 | 175.18 | 8.1 | 86.7 | 179.0 | 6.5 | 88.6 |
| CC standard-3 | 80.64 | 78.57 | 5.5 | 97.5 | 91.6 | 9.1 | 113.7 |
| CC standard-4 | 30.64 | 32.63 | 5.5 | 106.6 | 32.3 | 5.9 | 105.7 |
| CC standard-5 | 10.11 | 11.24 | 12.6 | 114.7 | 9.9 | 15.4 | 100.6 |
| CC standard-6 | 3.03 | 3.18 | 12.4 | 102.6 | 2.9 | 1.7 | 92.6 |
| CC standard-7 | 1.06 | 1.07 | 5.1 | 97.3 | 1.1 | 4.9 | 102.2 |
| CC standard-8 | 0.53 | 0.51 | 3.8 | 96.3 | 0.47 | 10.3 | 88.7 |
| HQC | 504.00 | 483.33 | 5.3 | 95.9 | 483.3 | 5.3 | 95.9 |
| MQC | 80.64 | 70.71 | 13.5 | 87.7 | 71.7 | 13.6 | 88.9 |
| LQC | 3.03 | 2.64 | 23.3 | 87.0 | 2.82 | 16.3 | 93.2 |
| LLOQ QC | 1.06 | 1.22 | 31.5 | 114.8 | 1.16 | 27.8 | 109.1 |
| Nornicotine | |||||||
| CC standard-1 | 504.00 | 544.00 | 3.8 | 107.9 | 527.2 | 7.3 | 104.6 |
| CC standard-2 | 201.60 | 179.10 | 6.5 | 88.8 | 179.2 | 6.4 | 88.9 |
| CC standard-3 | 80.64 | 90.38 | 8.9 | 112.1 | 90.7 | 9.3 | 112.4 |
| CC standard-4 | 30.64 | 32.27 | 5.9 | 105.3 | 32.5 | 5.9 | 105.8 |
| CC standard-5 | 10.11 | 10.19 | 14.4 | 100.7 | 10.3 | 13.1 | 102.3 |
| CC standard-6 | 3.03 | 2.99 | 2.2 | 98.6 | 3.0 | 1.6 | 99.1 |
| CC standard-7 | 1.06 | 1.08 | 6.2 | 101.8 | 1.08 | 6.2 | 101.8 |
| CC standard-8 | 0.53 | 0.55 | 23.4 | 103.4 | 0.57 | 22.5 | 106.6 |
| HQC | 504.00 | 483.33 | 5.3 | 95.9 | 483.33 | 5.3 | 95.9 |
| MQC | 80.64 | 70.71 | 13.5 | 87.7 | 71.44 | 14.8 | 88.6 |
| LQC | 3.03 | 2.82 | 16.3 | 93.2 | 2.82 | 16.3 | 93.2 |
| LLOQ QC | 1.06 | 1.17 | 18.4 | 110.7 | 1.24 | 22.4 | 117.0 |
| Cotinine | |||||||
| CC standard-1 | 504.00 | 518.83 | 7.3 | 102.9 | 504.83 | 8.7 | 100.2 |
| CC standard-2 | 201.60 | 179.60 | 6.4 | 89.1 | 181.43 | 6.7 | 90.0 |
| CC standard-3 | 80.64 | 90.55 | 9.2 | 112.2 | 90.11 | 10.0 | 111.7 |
| CC standard-4 | 30.64 | 31.94 | 7.5 | 104.2 | 31.61 | 6.9 | 103.2 |
| CC standard-5 | 10.11 | 10.69 | 11.2 | 105.6 | 10.85 | 10.6 | 107.3 |
| CC standard-6 | 3.03 | 2.98 | 2.1 | 98.4 | 2.99 | 2.7 | 98.8 |
| CC standard-7 | 1.06 | 1.06 | 6.8 | 100.3 | 1.09 | 8.4 | 102.5 |
| CC standard-8 | 0.53 | 1.49 | 14.8 | 81.7 | 1.49 | 148.0 | 281.8 |
| HQC | 504.00 | 480.83 | 5.1 | 95.4 | 478.00 | 4.6 | 94.8 |
| MQC | 80.64 | 70.61 | 12.4 | 87.6 | 72.44 | 7.4 | 89.8 |
| LQC | 3.03 | 2.84 | 14.6 | 93.9 | 2.84 | 14.5 | 93.7 |
| LLOQ QC | 1.06 | 1.19 | 18.7 | 112.3 | 1.21 | 17.6 | 114.5 |
| Norcotinine | |||||||
| CC standard-1 | 504.00 | 545.67 | 3.8 | 108.3 | 545.67 | 3.8 | 108.3 |
| CC standard-2 | 201.60 | 180.60 | 6.0 | 89.6 | 180.60 | 6.0 | 89.6 |
| CC standard-3 | 80.64 | 92.61 | 8.0 | 114.8 | 93.94 | 8.0 | 116.5 |
| CC standard-4 | 30.64 | 32.44 | 6.6 | 105.9 | 31.44 | 6.7 | 102.6 |
| CC standard-5 | 10.11 | 10.19 | 14.4 | 100.7 | 10.35 | 14.5 | 102.4 |
| CC standard-6 | 3.03 | 2.98 | 2.0 | 98.2 | 2.99 | 1.6 | 98.8 |
| CC standard-7 | 1.06 | 0.99 | 7.3 | 93.1 | 1.02 | 11.2 | 96.2 |
| CC standard-8 | 0.53 | 0.64 | 19.9 | 119.8 | 0.62 | 17.9 | 117.3 |
| HQC | 504.00 | 478.00 | 4.7 | 94.8 | 484.50 | 3.7 | 96.1 |
| MQC | 80.64 | 74.27 | 6.1 | 92.1 | 77.27 | 8.6 | 95.8 |
| LQC | 3.03 | 2.95 | 4.0 | 97.5 | 3.00 | 1.9 | 99.1 |
| LLOQ QC | 1.06 | 1.19 | 18.7 | 112.3 | 1.17 | 15.9 | 110.7 |
| Trans-3′-hydroxy cotinine | |||||||
| CC standard-1 | 504.00 | 511.67 | 7.4 | 101.5 | 529.17 | 7.8 | 105.0 |
| CC standard-2 | 201.60 | 183.10 | 6.0 | 90.8 | 182.43 | 6.1 | 90.5 |
| CC standard-3 | 80.64 | 89.61 | 9.4 | 111.1 | 90.27 | 10.2 | 111.9 |
| CC standard-4 | 30.64 | 30.94 | 7.4 | 101.0 | 31.44 | 6.7 | 102.6 |
| CC standard-5 | 10.11 | 11.02 | 9.8 | 109.0 | 10.69 | 12.7 | 105.7 |
| CC standard-6 | 3.03 | 2.98 | 2.9 | 98.2 | 3.01 | 2.2 | 99.3 |
| CC standard-7 | 1.06 | 1.05 | 7.4 | 99.4 | 1.07 | 10.9 | 100.9 |
| CC standard-8 | 0.53 | 2.39 | 116.8 | 451.6 | 0.61 | 19.3 | 115.1 |
| HQC | 504.00 | 484.17 | 3.7 | 96.1 | 484.17 | 3.7 | 96.1 |
| MQC | 80.64 | 73.61 | 7.4 | 91.3 | 75.44 | 10.7 | 93.6 |
| LQC | 3.03 | 3.02 | 0.4 | 99.8 | 3.00 | 1.9 | 98.9 |
| LLOQ QC | 1.06 | 1.12 | 9.2 | 106.0 | 1.11 | 10.1 | 105.0 |
CC, calibration curve.
Recovery, Matrix Effect, and Stability of Analytes in Plasma from Nonsmokers.
Recovery of nicotine and its metabolites was estimated by analyses of two sets of six replicates of extracted plasma at low, middle, and high QC standards and postspiked (represent 100% recovery) samples along with IS (Table 2). The three levels of recovered QC standards were prepared in blank extracted plasma from nonsmokers. The aqueous dilutions were postspiked in nonsmoker blank plasma sample to get the same concentrations (504.0, 80.6, and 3.03, ng/ml). An overall extraction recovery was determined by comparing the mean peak area ratios of the analytes with the IS obtained from the extracted QC (matrix samples from nonsmokers versus the unextracted standards).
TABLE 2.
Recovery and matrix effect of four levels of QC standards of nicotine, nornicotine, cotinine, norcotinine, and trans-3′-hydroxycotinine in pooled human plasma from HIV-negative nonsmokers
| Analyte | Nominal Concentration | Recovery (n = 6) |
Matrix Effect (n = 6) |
||
|---|---|---|---|---|---|
| CV | Recovery | CV | Matrix Effect | ||
| ng/ml | % | ||||
| Nicotine | |||||
| HQC | 504.00 | 5.6 | 97.9 | 5.7 | 97.6 |
| MQC | 80.64 | 8.4 | 102.9 | 8.3 | 102.5 |
| LQC | 3.03 | 24.4 | 95.7 | 27.2 | 91.6 |
| Nornicotine | |||||
| HQC | 504.00 | 8.6 | 98.5 | 9.8 | 100.9 |
| MQC | 80.64 | 8.3 | 102.5 | 8.9 | 101.1 |
| LQC | 3.03 | 22.9 | 86.7 | 23.6 | 86.1 |
| Cotinine | |||||
| HQC | 504.00 | 9.7 | 100.9 | 5.6 | 97.9 |
| MQC | 80.64 | 9.1 | 99.5 | 8.4 | 102.9 |
| LQC | 3.03 | 29.3 | 89.6 | 24.4 | 95.7 |
| Norcotinine | |||||
| HQC | 504.00 | 9.7 | 100.9 | 9.6 | 101.2 |
| MQC | 80.64 | 9.1 | 99.5 | 8.7 | 100.2 |
| LQC | 3.03 | 29.3 | 89.6 | 21.0 | 83.2 |
| Trans-3′-hydroxy cotinine | |||||
| HQC | 504.00 | 8.7 | 104.2 | 9.7 | 103.2 |
| MQC | 80.64 | 11.3 | 102.8 | 9.0 | 106.2 |
| LQC | 3.03 | 23.5 | 86.6 | 15.7 | 90.5 |
CC, calibration curve.
The matrix effect of nicotine and its metabolites along with IS was evaluated by analyzing two sets of six replicates of each low, middle, and high QC standards (LQC, MQC, and HQC, respectively) from postspiked (extracted blank plasma samples) and spiked standards in aqueous solutions (represent no matrix effect) (Table 2). Ninety blank matrix samples from nonsmokers and 18 samples of each analyte were processed and extracted as described above. The aqueous stock QC dilutions (low, middle, and high) were spiked in the extracted blank samples to obtain the QC standards (504.0, 80.6, and 3.03 ng/ml). Similarly, these QC standards were prepared by spiking analytes in reconstitution solution to obtain the same concentrations. Matrix ion suppression was calculated by comparing the mean peak area ratios of each analyte and IS generated from the postspiked QC standards from plasma samples from nonsmokers with reconstituted spiked QC standards. A relative matrix effect was estimated by comparing the mean peak area ratios of the analytes to IS obtained from the postspiked QC.
Six replicates of stability samples at concentrations of 504.0, 80.64, and 3.03 ng/ml were prepared for each analyte in pooled plasma from the nonsmokers (Table 3). The samples were stored at −80°C for several weeks to estimate the degradation of analyte in the matrix. Stability QC samples were extracted along with freshly prepared calibration standards in pooled plasma from the nonsmokers. The stability samples were frozen and stored for 6 months at −80°C. Stability QC samples were freeze-thawed for three cycles, and analyzed with freshly prepared calibration curve standards. The linearity of the analysis was determined using freshly spiked calibration standards, which were analyzed in duplicate along with stability samples (bench top, freeze-thaw stability) processed as previously described (Earla et al., 2010).
TABLE 3.
Benchtop (25°C) and four cycle freeze-thaw (−80°C) stability QC standards of nicotine, nornicotine, cotinine, norcotinine, and trans-3′-hydroxycotinine in pooled human plasma from HIV-negative nonsmoker individuals.
| Analyte | Nominal Concentration | Bench Top (n = 6) |
Freeze-Thaw (n = 6) |
||||
|---|---|---|---|---|---|---|---|
| Calculated Concentration | CV | Stability | Calculated Concentration | CV | Stability | ||
| ng/ml | ng/ml | % | ng/ml | % | |||
| Nicotine | |||||||
| HQC | 504.00 | 511.71 | 9.3 | 101.5 | 512.01 | 9.2 | 101.6 |
| MQC | 80.64 | 83.19 | 7.1 | 103.2 | 82.89 | 6.8 | 102.8 |
| LQC | 3.03 | 2.56 | 13.3 | 88.6 | 2.68 | 15.2 | 93.5 |
| Nornicotine | |||||||
| HQC | 504.00 | 564.28 | 22.8 | 112.0 | 574.01 | 20.3 | 113.9 |
| MQC | 80.64 | 79.67 | 14.2 | 98.9 | 78.26 | 15.7 | 97.1 |
| LQC | 3.03 | 3.00 | 10.3 | 101.9 | 2.78 | 15.0 | 96.5 |
| Cotinine | |||||||
| HQC | 504.00 | 523.86 | 8.4 | 103.9 | 562.01 | 19.8 | 111.5 |
| MQC | 80.64 | 81.87 | 6.6 | 101.6 | 76.36 | 14.8 | 94.8 |
| LQC | 3.03 | 2.49 | 14.7 | 87.6 | 2.67 | 14.7 | 94.0 |
| Norcotinine | |||||||
| HQC | 504.00 | 528.38 | 13.8 | 104.8 | 577.01 | 19.7 | 114.5 |
| MQC | 80.64 | 83.19 | 14.2 | 103.2 | 81.36 | 14.4 | 101.0 |
| LQC | 3.03 | 2.69 | 13.2 | 94.5 | 3.00 | 14.5 | 104.8 |
| Trans-3′-hydroxy cotinine | |||||||
| HQC | 504.00 | 507.28 | 9.9 | 100.7 | 520.56 | 9.6 | 103.3 |
| MQC | 80.64 | 82.37 | 7.3 | 102.3 | 78.28 | 13.5 | 97.1 |
| LQC | 3.03 | 2.76 | 15.2 | 95.5 | 2.69 | 14.0 | 94.5 |
CC, calibration curve.
Patient Recruitment.
After we received institutional review board approval from the University of Missouri-Kansas City and Cameroonian Ministry of Health Regional Hospital in Bamenda, Cameroon, we recruited our subjects in Cameroon, Africa, using strict inclusion and exclusion criteria. For the smokers category, we recruited 17 volunteers (12 men and 5 women) who reported a smoking history of at least one to two packs per day for the past 3 years. For the HIV-1–positive smoker category, we recruited six individuals (five men and one woman) with CD4 counts ranging between 100 and 500 cells/µl except one subject with a similar smoking history. The demographics, including packs per day and duration of smoking, are similar in both groups, except for diagnostic dates of infection for the HIV-1–positive smoker category. Among the HIV-1–positive patients, patients 1 and 3 have AIDS as shown by a decrease in the CD4 count below 200 cells/µl (Table 4). The male/female ratio is relatively high in both categories, owing to the low prevalence of smoking among women in Cameroon, especially in the HIV-1–infected population. This study excluded pregnant women, individuals aged <21 years or >65 years, patients with either hepatitis B, malaria, or tuberculosis, a CD4 count <100 or >500 CD4 lymphocytes/µL for the HIV-1–positive group, and those receiving either antiretroviral drugs, traditional medicines, or over-the-counter drugs. We conducted the clinical screening tests for malaria, hepatitis, and tuberculosis infections using standard procedures as described (Bodelle et al., 2004; Yamaguchi et al., 2004, 2006; Vallari et al., 2010). Upon recruitment of HIV-1-positive and HIV-negative smokers, 60 ml blood was drawn from each individual followed by determination of the participant’s CD4 counts (Table 4) using BD FACSCount instrument (BD East Africa Ltd, Nairobi, Kenya) at Provincial Regional Hospital, Bamenda, Cameroon. The viral load of HIV-1–infected smokers was determined in plasma using real-time reverse-transcription polymerase chain reaction by a Roche Amplicor System (Biocentric) at the Pasteur Center (Bamenda, Cameroon). The remaining plasma samples were shipped to our laboratory in Kansas City, Missouri, and stored at −80°C until further analysis. To optimize the method, six control samples were also collected from nonsmoker healthy volunteers at the University of Missouri-Kansas City (four men, two women) ranging in age from 25 to 45 years. HIV-1–positive and HIV-negative smoker plasma samples were processed and extracted according to the above-described sample preparation and extraction protocols.
TABLE 4.
Determination of nicotine, nornicotine, cotinine, norcotinine, and trans-3′-hydroxycotinine in plasma from HIV-1–positive and HIV-negative smokers
| Patient | CD4 Count | Nicotine | Cotinine | Trans-3′-Hydroxycotinine | Nornicotine | Norcotinine |
|---|---|---|---|---|---|---|
| ng/ml | ||||||
| HIV-negative smokers | ||||||
| 1 | 1416 | 10.87 | 2.33 | 2.28 | 4.10 | 0.88 |
| 2 | 938 | 33.07 | 115.55 | 68.19 | 3.97 | 2.45 |
| 3 | 1488 | 14.85 | 123.38 | 24.33 | 2.15 | 1.61 |
| 4 | 1289 | 27.49 | 84.93 | 4.86 | 1.65 | 1.19 |
| 5 | 742 | 28.96 | 79.51 | 94.19 | 3.61 | 3.28 |
| 6 | 936 | 53.06 | 65.49 | 3.43 | 2.91 | 0.63 |
| 7 | 1438 | 61.59 | 119.04 | 71.65 | 1.91 | 3.87 |
| 8 | 1039 | 51.45 | 98.37 | 33.28 | 1.64 | 2.42 |
| 9 | 500 | 17.10 | 23.59 | 1.50 | 2.46 | 0.87 |
| 10 | 700 | 31.86 | 38.45 | 18.86 | 2.94 | 1.17 |
| 11 | 921 | 36.91 | 98.02 | 20.12 | 1.09 | 1.15 |
| 12 | 1032 | 40.11 | 106.47 | 19.42 | 1.26 | 1.02 |
| 13 | 1382 | 49.78 | 117.30 | 65.88 | 1.52 | 2.11 |
| 14 | 594 | 18.90 | 33.68 | 8.02 | 1.70 | 0.84 |
| 15 | 838 | 39.76 | 69.86 | 8.09 | 4.05 | 1.47 |
| 16 | 977 | 10.85 | 4.57 | 3.31 | 0.58 | 0.54 |
| 17 | 1009 | 39.36 | 93.30 | 56.26 | 0.76 | 2.40 |
| Mean ± S.D. | 33.29 ± 15.414 | 74.93 ± 40.526 | 29.63 ± 29.853 | 2.24 ± 1.187 | 1.62 ± 0.987 | |
| HIV-1–positive smokers | ||||||
| 1 | 110 | 10.50 | 197.00 | 115.60 | 5.30 | 5.50 |
| 2 | 584 | 6.50 | 56.00 | 34.10 | 4.70 | 1.60 |
| 3 | 13 | 1.90 | 75.80 | 12.20 | 4.50 | 1.30 |
| 4 | 540 | 12.30 | 85.40 | 14.00 | 5.70 | 1.40 |
| 5 | 412 | 7.20 | 95.10 | 22.50 | 8.40 | 2.20 |
| 6 | 235 | 4.60 | 16.10 | 1.90 | 12.00 | 0.60 |
| Mean ± S.D. | 7.17 ± 3.801 | 87.57 ± 64.464 | 33.38 ± 41.699 | 6.77 ± 2.924 | 2.10 ± 1.746 | |
Statistical Analysis.
The concentration of all analytes in the plasma from subjects was calculated by Analyst software (AB Sciex). Statistical significance (P values) was calculated using one-way analysis of variance and the mean and standard deviations were calculated using Microsoft Excel software (Microsoft Corporation, Redmond, WA). The LC-MS/MS data on nicotine, cotinine, trans-3′-hydroxycotinine, nornicotine, and norcotinine obtained from HIV-1–positive and HIV-negative smokers were analyzed using box plot statistical software (SPSS Inc, an IBM Company, Chicago, IL).
Results
Tandem Mass Spectrometry.
The most stable precursor ion with proton adducts [M+H]+ for nicotine, nornicotine, nicotine-d4, cotinine, norcotinine, trans-3′-hydroxycotinine, and cotinine-d3 was optimized by adjusting the declustering potential at 36, 36, 36, 46, 51, 51, and 46 V, respectively. The collision energy was used to optimize the product ions of nicotine, nornicotine, nicotine-d4, cotinine, norcotinine, trans-3′-hydroxycotinine, and cotinine-d3 at 33, 17, 35, 23, 25, 25, and 29 V, respectively (Supplemental Table 1). The selected appropriate MRM transitions for quantitative analysis of nicotine and its metabolites are shown in Fig. 1 and Supplemental Table 1.
System Suitability and Carry-Over Tests.
The system suitability test resulted in <2% variation for nicotine and its metabolites. The %CV for nicotine and nornicotine with nicotine-d4 as IS were 0.72 and 1.97, respectively (Supplemental Table 2). Similarly, the %CV for cotinine, trans-3′-hydroxycotinine, and norcotinine with their IS cotinine-d3 were 1.01, 0.77, and 0.31, respectively. The carry-over test for nicotine and its metabolites at the highest calibrator did not show any carry-over to the blank sample.
Specificity, Selectivity, Limit of Quantification, and Linearity of Calibration Standards.
Nicotine and its metabolites, as well as IS, were separated from endogenous interference peaks of the blank plasma matrix (Supplemental Fig. 1). Fig. 2, A and B, shows the extracted chromatogram peaks for LLOQ and ULOQ with IS. The LLOQ for nicotine and its metabolites in plasma was obtained at 0.53 ng/ml. The mean LLOQ peak areas for nicotine, cotinine, trans-3′-hydroxycotinine, nornicotine, and norcotinine were 1254, 4902, 941, 481, and 1689, respectively. Similarly, the mean ULOQ peak area for nicotine, cotinine, trans-3′-hydroxycotinine, nornicotine, and norcotinine were 153,522, 1,293,454, 523,735, 134,858, and 575,084, respectively. Fig. 2, C–E, represents an example of a QC extracted sample validation of LQC (3.03 ng/ml), MQC (80.64 ng/ml), and HQC (504 ng/ml) chromatogram peak responses of nicotine, its metabolites, and IS, respectively, in control plasma. The coefficient of determination (R2) for nicotine and its metabolites ranged from 0.9963 to 0.9994 (Supplemental Fig. 2).
Accuracy and Precision.
The best linear fit and least-square residuals for the standards were achieved with a 1/X weighing factor that yielded a mean linear regression equation for the calibration curve. The assay was linear over the range of 0.53–504 ng/ml with R2 ≥ 0.996 (n = 6). A regression equation and coefficient of determination were obtained as follows (Supplemental Fig. 2): nicotine, y = 0.0003x + 0.0035; trans-3′-hydroxycotinine, y = 0.0068x + 0.0055; nornicotine, y = 0.0009x + 0.0073; cotinine, y = 0.0103x − 0.0289; and norcotinine, y = 0.007x + 0.024. The percentages of accuracy at LLOQ between the days were as follows: 109.1%–114.8% for nicotine, 110.7%–117% for nornicotine, 112.3%–114.5% for cotinine, 110.7%–112.3% for norcotinine, and 105%–106% for trans-3′-hydroxycotinine (Supplemental Table 2).
Recovery, Matrix Effect, and Stability of Analytes in Plasma from Nonsmokers.
The percentages of recovery of nicotine, cotinine, trans-3′-hydroxycotinine, nornicotine, and norcotinine were as follows: 95.7%–102.9%, 89.6%–100.9%, 86.6%–104.2%, 86.7%–102.5%, and 89.6%–100.9%, respectively (Table 2). Six replicates of LQC, MQC, and HQC were tested for a matrix effect. The percentages of matrix effects were 91.6%–102.5%, 95.7%–102.9%, 90.5%–106.2%, 86.1%–101.1%, and 83.2%–101.2% for nicotine, cotinine, trans-3′-hydroxycotinine, nornicotine, and norcotinine, respectively (Table 2). The stability results, which are presented as the percentage of stability (Table 3), yielded bench-top stability of <13% and freeze-thaw stability of approximately 14% for nicotine and its metabolites. The degradation of nicotine and its metabolites was <15% at LQC, MQC, and HQC levels.
Quantitative Estimation of Nicotine and its Metabolites in Plasma.
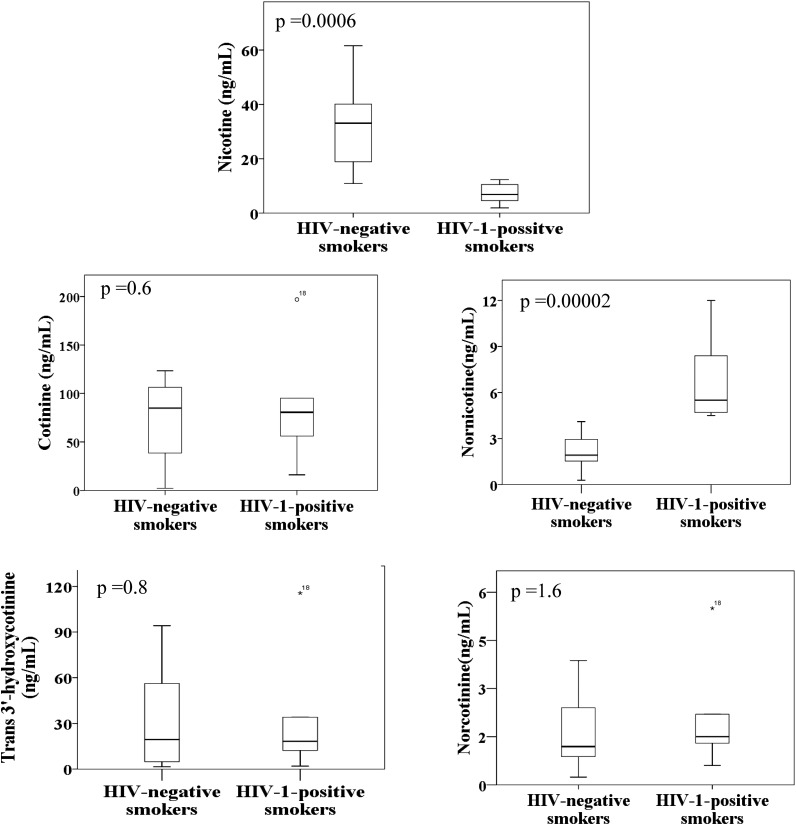
Nicotine and its metabolites cotinine, trans-3′-hydroxycotinine, nornicotine, and norcotinine were determined at nanogram per milliliter levels in the plasma of both HIV-1–positive and HIV-negative smokers (Table 4). Nicotine concentration in the HIV-negative smokers ranged from 10.87 to 61.59 ng/ml, whereas nicotine concentration in the HIV-1–positive smokers ranged from 1.9 to 12.3 ng/ml. The concentrations of cotinine, trans-3′-hydroxycotine, nornicotine, and norcotinine were 2.3–123.38 ng/ml, 1.5–94.19 ng/ml, 1.09–4.10 ng/ml, and 0.63–3.87 ng/ml, respectively, in HIV-negative smokers. However, the concentrations of cotinine, trans-3′-hydroxycotinine, nornicotine, and norcotinine concentrations were 56.00–197.00 ng/ml, 12.20–115.60 ng/ml, 4.50–5.70 ng/ml, and 1.30–5.50 ng/ml, respectively in HIV-1–positive smokers (Table 4). There were no significant differences in the concentration of nicotine and its metabolites between male and female subjects when analyzed for HIV-negative smokers (data not shown). The mean nicotine concentration in the plasma of HIV-1–positive smokers (7.2 ± 3.80 ng/ml) was 5-fold lower than HIV-negative smokers (33.3 ± 15.45 ng/ml) (Fig. 3; Table 4). The mean concentration of nicotine metabolite nornicotine was 3-fold higher in HIV-1–positive smokers (6.8 ± 2.93 ng/ml) than in HIV-negative smokers (2.2 ± 1.21 ng/ml). Although it was not statistically significant, the mean concentration of cotinine was also higher in HIV-1–positive smokers (87.5 ± 64.46 ng/ml) than in HIV-negative smokers (74.9 ± 40.45 ng/ml). However, the mean concentrations of other nicotine metabolites between HIV-negative and HIV-1–positive smokers were not altered (Fig. 3; Table 4).
Fig. 3.
Levels of nicotine, cotinine, trans-3′-hydroxycotinine, nornicotine, and norcotinine in the plasma of HIV-1–positive (n = 6) and HIV-negative smokers (n = 17) presented in a box plot created with SPSS software. The box plot shows distribution of all individuals, median, first quartile below the median, third quartiles above the median, and outliers in plasma from both HIV-1–positive smokers and HIV-negative smokers.
Discussion
In this study, we have developed a simple, fast, and sensitive ESI-LC-MS/MS analytical method to simultaneously determine the concentrations of nicotine and four of its metabolites in plasma samples. This method enables the determination of concentrations of nicotine and its metabolites in HIV-1–positive and HIV-negative smokers. To our knowledge, this is the first report demonstrating the application of an ESI-LC-MS/MS method for concurrent determination of the concentrations of nicotine and four of its metabolites with a sensitivity of approximately 0.5 ng/ml. Of importance, this is also the first report to show the difference in the concentrations of nicotine and its metabolites in HIV-1–positive smokers compared with HIV-negative smokers.
LC-MS/MS bioanalytical results are generally frequently inconsistent because of ineffective sample preparation and extraction methods. These problems, however, can be eliminated by modifying sample extraction techniques and mass spectrometry parameters including MRM transitions. Liquid–liquid and solid phase extractions are generally the most effective approach. However, they are expensive and time-consuming (Earla et al., 2012). The elimination of unwanted water-soluble compounds, such as phosphates and sulfates from plasma, is important in ESI techniques. Zinc sulfate and phosphate present in the plasma has been shown to cause ion suppression of analytes, leading to inconsistent results during the ESI analysis (Bogusz et al., 2007; Clavijo et al., 2009; Earla et al., 2012). In addition to extraction and sample preparation during the LC-MS/MS analysis, pH of the reconstitution solution and mobile phase are important factors to achieve optimal chromatographic separation, peak resolution, reproducibility, and reliability. Furthermore, reconstitution of the solution also plays an important role in the ionization of analytes in the ESI method. By considering the above array of parameters, we have developed a simple and rapid ESI–LC-MS/MS technique to simultaneously estimate nicotine and its four metabolites in plasma by using strong cation exchange SPE.
The system suitability test results showed that the method is consistent and reproducible for all analytes. Carry-over test results showed that there is no significant carry-over to confound results of the next samples analyzed. The assay conditions had adequate specificity for nicotine and its metabolites, and no interfering peaks were observed with retention times coinciding with the peak of interest. The signal-to-noise ratio of all analytes was measured at the LLOQ level and was found to be >5-fold higher than that of the extracted blank peak area. A high signal-to-noise ratio suggests a relatively high quality of extraction efficiency and selectivity, with minimal plasma endogenous interference. The chromatogram peak response and intensity of nicotine and its metabolites were proportional to the concentrations from LLOQ to ULOQ with two ISs, which strongly suggests that the method is reproducible and robust. Moreover, this method was relatively more sensitive than the other methods, because the LLOQ (0.53 ng/ml) for nicotine and its metabolites was much higher than the LLOQ in other reported methods (2–10 ng/ml) (Byrd et al., 2005; Shakleya and Huestis, 2009; Miller et al., 2010; Shu and Wang, 2013). More importantly, this level of sensitivity was obtained with five analytes simultaneously as opposed to the reported methods, in which the methods were developed with two to three analytes simultaneously. Moreover, the optimization of LLOQ and extraction are difficult using the plasma matrix compared with other matrices because plasma has more endogenous interferences than many other matrices.
The results of the accuracy and precision chromatograms suggest that the method is robust, and precise for the analysis of nicotine and its metabolites in the plasma. All previously reported LC-MS/MS methods have used ammonium acetate or ammonium formate buffers in their mobile phase systems with a proton adducts in negative and positive modes (Bogusz et al., 2007; Miller et al., 2010). Such ammoniated buffer mobile phase systems may enhance ionization of analytes and clog the peak tubes, pump seals, and precipitate in the flow line of the mobile phase. This would lead to an increase in backup pressure in the HPLC column causing leakage of the samples. Therefore, the optimization of nicotine and its metabolites in a formic acid mobile phase system with a proton adduct [M+H]+ in the positive ion mode is considered superior to the other mobile phase. Formic acid–containing mobile phases have the advantage over the ammoniated buffer mobile phase systems, because the former mobile phase system does not obstruct the flow and maintains a consistent pressure in the HPLC column (Earla et al., 2012). Second, this mobile phase system is easy to clean, owing to lack of precipitation. The nicotine and its metabolites were all eluted from the column within the same retention time (approximately 2 minutes) showing a sharp resolution peak. The analytes were separated and quantified based on their m/z, which is relatively fast and efficient compared with other reported methods (Kataoka et al., 2009; Miller et al., 2010).
The recovery efficiency of this method is higher than the previous methods (Miller et al., 2010; Jacob et al., 2011; Ande et al., 2012; Jin et al., 2012a), and the peak response is also reproducible. We previously developed a liquid–liquid extraction method for nicotine and its metabolites in astrocytic and monocytic cell lines (Ande et al., 2012; Jin et al., 2012a). However, in the present method, we used strong cation exchange SPE cartridges for plasma sample analysis. Plasma has numerous complex endogenous interfering components compared with astrocytes and monocytes matrices. During optimization of the plasma extraction, we tested several other SPE techniques using different solvent conditions and elution methods such as strong anion SPE and lipophilic-hydrophilic balance SPE. The current method is more sensitive than other reported methods (Marclay and Saugy, 2010; Miller et al., 2010), and is able to quantify nicotine and its metabolites at the LLOQ level without any interference, with high reproducibility and high accuracy. In the currently described sample preparation technique, all plasma samples including controls, standards, and test samples were pretreated with aqueous formic acid solution to make them more acidic prior to SPE. Under acidic conditions we found the results were consistent without any interference. The nicotine and its four metabolites were stable for 48 weeks with three freeze-thaw cycles at −80°C and for 5 hours at ambient temperature.
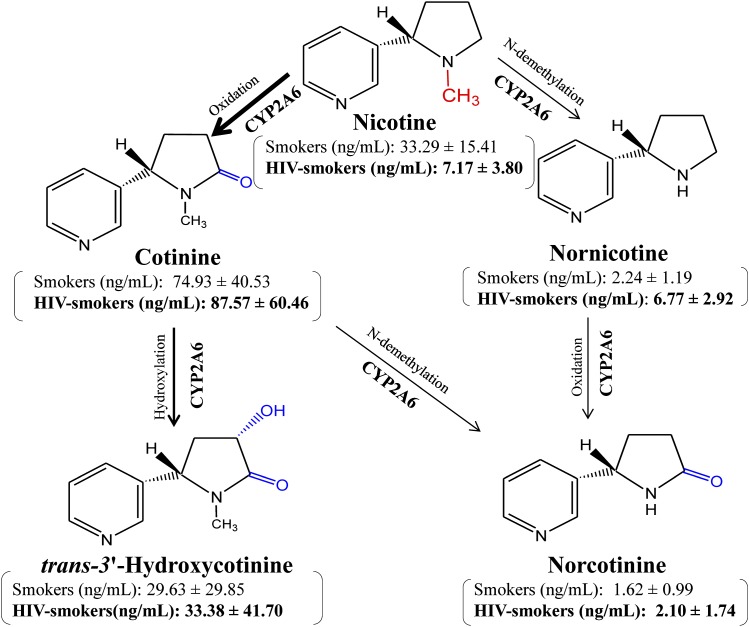
Using our newly developed LC-MS/MS technique, we analyzed nicotine and its metabolites in the plasma of HIV-1–positive and HIV-negative smokers. These individuals were recruited with strict exclusion criteria to reduce the possibility of confounding by other drugs or comorbidities. The exclusion criteria were critical to determine the specific effects of mild to moderate smoking in HIV-1–positive patients without any interference by antiretroviral therapy, other medications, or other substances of abuse. The nicotine level is approximately 5-fold lower in HIV-1–positive smokers than in HIV-negative smokers, strongly suggesting that HIV-1 infection increases the metabolism of nicotine (Fig. 4). This hypothesis is further strengthened by the findings that the levels of nicotine metabolites, nornicotine, and, to some extent, cotinine are relatively higher in HIV-1–positive than HIV-negative smokers. CYP2A6 is known to metabolize nicotine into the major metabolite cotinine and other minor metabolites including nornicotine (Benowitz and Jacob, 1994) (Fig. 4). Cotinine is further metabolized into trans-3′-hydroxycotinine, norcotinine, and other minor metabolites by CYP2A6 (Hecht et al., 2000; Levi et al., 2007).
Fig. 4.
The metabolism of nicotine representing the concentrations of nicotine and its metabolites. The remaining mean relative amounts ± S.D. (in nanograms per milliliter) of nicotine, cotinine, nornicotine, norcotinine, and trans-3′-hydroxycotinine found in the plasma of HIV-1–positive smokers (n = 6) and HIV-negative smokers plasma (n = 17) are shown in parentheses. The intensity of arrows suggests relative contribution and amounts of CYP2A6-mediated pathways for nicotine metabolism. The nicotine is mainly metabolized to cotinine followed by trans-3′-hydroxycotinine. In addition, nicotine is also metabolized by other pathways to nornicotine and norcotinine. Norcotinine is also formed from cotinine. These are standard chemicals and were made using ChemDraw Ultra (version 6.0.1; CambridgeSoft.com).
Collectively, these results suggest that nicotine metabolism is enhanced significantly in HIV-1–positive smokers compared with HIV-negative smokers. Enhanced nicotine metabolism is known to increase ROS and reactive metabolites, leading to increased cell toxicity and cancer of the lungs, esophagus, and liver (Ande et al., 2013). This may lead to an increased risk of cancer in HIV-1–positive smokers compared with HIV-negative smokers. Indeed, our recent study showed an increase in oxidative DNA damage and viral load in the plasma of HIV-1-positive smokers compared to HIV-1-positive non-smokers (data not shown; Ande et al., 2013). We also reported enhanced oxidative stress that was correlated with an increase in the viral load in HIV-1–positive smokers compared with HIV-1–positive nonsmokers (Ande et al., 2013). Previous reports also suggest a role of smoking in increased viral replication and a possible association with increased oxidative stress (Boelaert et al., 1996; Israel and Gougerot-Pocidalo, 1997; Aquaro et al., 2007).
Since the prevalence of smoking is higher in HIV-1–positive individuals than in the general population (Burkhalter et al., 2005) and smoking is in turn associated with increased viral replication (Abbud et al., 1995; Rock et al., 2008; Zhao et al., 2010), it is important to understand the underlying mechanism of smoking/nicotine-mediated HIV-1 replication. This report suggests a role of nicotine metabolism in the oxidative stress–mediated increase in viral replication in HIV-1–positive smokers. However, further studies are necessary to elucidate this mechanism to confirm the role of CYP2A6-mediated nicotine metabolism, especially in monocytes/macrophages, in HIV-1 replication. Future studies in these patients will be directed toward determining the expression level and activity of CYP2A6 in monocytes/macrophages.
Supplementary Material
Acknowledgments
The authors are deeply grateful for the assistance and experience of Dr. Paul Ngang Achu and Annette Njinda at the Mezam Polyclinic HIV/AIDS Treatment Center. The authors also thank Dr. Charles Awasom, director, and Dr. Leo Ayuk, director of infectious diseases, at the Regional Hospital in Cameroon. Finally, none of this research would have been possible without the generous participation and interest of our many patients there.
Abbreviations
- CV
coefficient of variation
- ESI
electrospray ionization
- HPLC
high-performance liquid chromatography
- HQC
high quality control
- IS
internal standard
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- LLOQ
lower limit of quantitation
- LQC
low quality control
- MQC
middle quality control
- MRM
multiple reaction monitoring
- m/z
mass-to-charge ratio
- QC
quality control
- ROS
reactive oxygen species
- SPE
solid phase extraction
- ULOQ
upper limit of quantitation
Authorship Contributions
Participated in research design: Earla, McArthur, A. Kumar, S. Kumar.
Conducted experiments: Earla, Ande, McArthur.
Contributed new reagents or analytic tools: McArthur, S. Kumar.
Performed data analysis: Earla, Ande, S. Kumar.
Wrote or contributed to the writing of the manuscript: Earla, Ande, McArthur, A. Kumar, S. Kumar.
Footnotes
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grant R21 DA031616] and the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant R01 AA022063].
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Abbud RA, Finegan CK, Guay LA, Rich EA. (1995) Enhanced production of human immunodeficiency virus type 1 by in vitro-infected alveolar macrophages from otherwise healthy cigarette smokers. J Infect Dis 172:859–863 [DOI] [PubMed] [Google Scholar]
- Ande A, Earla R, Jin M, Silverstein PS, Mitra AK, Kumar A, Kumar S. (2012) An LC-MS/MS method for concurrent determination of nicotine metabolites and the role of CYP2A6 in nicotine metabolite-mediated oxidative stress in SVGA astrocytes. Drug Alcohol Depend 125:49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ande A, McArthur C, Kumar A, Kumar S. (2013) Tobacco smoking effect on HIV-1 pathogenesis: role of cytochrome P450 isozymes. Expert Opin Drug Metab Toxicol 9:1453–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquaro S, Muscoli C, Ranazzi A, Pollicita M, Granato T, Masuelli L, Modesti A, Perno CF, Mollace V. (2007) The contribution of peroxynitrite generation in HIV replication in human primary macrophages. Retrovirology 4:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. (2008) Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther 83:531–541 [DOI] [PubMed] [Google Scholar]
- Benowitz NL. (2009) Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol 49:57–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd (1994) Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther 56:483–493 [DOI] [PubMed] [Google Scholar]
- Bernert JT, Jacob P, 3rd, Holiday DB, Benowitz NL, Sosnoff CS, Doig MV, Feyerabend C, Aldous KM, Sharifi M, Kellogg MD, et al. (2009) Interlaboratory comparability of serum cotinine measurements at smoker and nonsmoker concentration levels: a round-robin study. Nicotine Tob Res 11:1458–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer J, Peters FT, Kraemer T, Maurer HH. (2007) Detection and validated quantification of toxic alkaloids in human blood plasma—comparison of LC-APCI-MS with LC-ESI-MS/MS. J Mass Spectrom 42:621–633 [DOI] [PubMed] [Google Scholar]
- Bhagwat SV, Vijayasarathy C, Raza H, Mullick J, Avadhani NG. (1998) Preferential effects of nicotine and 4-(N-methyl-N-nitrosamine)-1-(3-pyridyl)-1-butanone on mitochondrial glutathione S-transferase A4-4 induction and increased oxidative stress in the rat brain. Biochem Pharmacol 56:831–839 [DOI] [PubMed] [Google Scholar]
- Bodelle P, Vallari A, Coffey R, McArthur CP, Beyeme M, Devare SG, Schochetman G, Brennan CA. (2004) Identification and genomic sequence of an HIV type 1 group N isolate from Cameroon. AIDS Res Hum Retroviruses 20:902–908 [DOI] [PubMed] [Google Scholar]
- Boelaert JR, Piette J, Weinberg GA, Sappey C, Weinberg ED. (1996) Iron and oxidative stress as a mechanism for the enhanced production of human immunodeficiency virus by alveolar macrophages from otherwise healthy cigarette smokers. J Infect Dis 173:1045–1047 [DOI] [PubMed] [Google Scholar]
- Bogusz MJ, Enazi EA, Hassan H, Abdel-Jawaad J, Ruwaily JA, Tufail MA. (2007) Simultaneous LC-MS-MS determination of cyclosporine A, tacrolimus, and sirolimus in whole blood as well as mycophenolic acid in plasma using common pretreatment procedure. J Chromatogr B Analyt Technol Biomed Life Sci 850:471–480 [DOI] [PubMed] [Google Scholar]
- Burkhalter JE, Springer CM, Chhabra R, Ostroff JS, Rapkin BD. (2005) Tobacco use and readiness to quit smoking in low-income HIV-infected persons. Nicotine Tob Res 7:511–522 [DOI] [PubMed] [Google Scholar]
- Byrd GD, Davis RA, Ogden MW. (2005) A rapid LC-MS-MS method for the determination of nicotine and cotinine in serum and saliva samples from smokers: validation and comparison with a radioimmunoassay method. J Chromatogr Sci 43:133–140 [DOI] [PubMed] [Google Scholar]
- Clavijo C, Strom T, Moll V, Betts R, Zhang YL, Christians U, Bendrick-Peart J. (2009) Development and validation of a semi-automated assay for the highly sensitive quantification of Biolimus A9 in human whole blood using high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 877:3506–3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earla R, Boddu SH, Cholkar K, Hariharan S, Jwala J, Mitra AK. (2010) Development and validation of a fast and sensitive bioanalytical method for the quantitative determination of glucocorticoids—quantitative measurement of dexamethasone in rabbit ocular matrices by liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal 52:525–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earla R, Cholkar K, Gunda S, Earla RL, Mitra AK. (2012) Bioanalytical method validation of rapamycin in ocular matrix by QTRAP LC-MS/MS: application to rabbit anterior tissue distribution by topical administration of rapamycin nanomicellar formulation. J Chromatogr B Analyt Technol Biomed Life Sci 908:76–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JG, Minkoff H, Schneider MF, Gange SJ, Cohen M, Watts DH, Gandhi M, Mocharnuk RS, Anastos K. (2006) Association of cigarette smoking with HIV prognosis among women in the HAART era: a report from the women’s interagency HIV study. Am J Public Health 96:1060–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS, Hochalter JB, Villalta PW, Murphy SE. (2000) 2′-Hydroxylation of nicotine by cytochrome P450 2A6 and human liver microsomes: formation of a lung carcinogen precursor. Proc Natl Acad Sci USA 97:12493–12497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël N, Gougerot-Pocidalo MA. (1997) Oxidative stress in human immunodeficiency virus infection. Cell Mol Life Sci 53:864–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, 3rd, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. (2011) Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B Analyt Technol Biomed Life Sci 879:267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MJ, Primatesta P, Erens B, Feyerabend C, Bryant A. (2003) Measuring nicotine intake in population surveys: comparability of saliva cotinine and plasma cotinine estimates. Nicotine Tob Res 5:349–355 [DOI] [PubMed] [Google Scholar]
- Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y. (1987) Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health 77:1435–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Arya P, Patel K, Singh B, Silverstein PS, Bhat HK, Kumar A, Kumar S. (2011) Effect of alcohol on drug efflux protein and drug metabolic enzymes in U937 macrophages. Alcohol Clin Exp Res 35:132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Earla R, Shah A, Earla RL, Gupte R, Mitra AK, Kumar A, Kumar S. (2012a) A LC-MS/MS method for concurrent determination of nicotine metabolites and role of CYP2A6 in nicotine metabolism in U937 macrophages: implications in oxidative stress in HIV + smokers. J Neuroimmune Pharmacol 7:289–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Kumar A, Kumar S. (2012b) Ethanol-mediated regulation of cytochrome P450 2A6 expression in monocytes: role of oxidative stress-mediated PKC/MEK/Nrf2 pathway. PLoS ONE 7:e35505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadlubar S, Anderson JP, Sweeney C, Gross MD, Lang NP, Kadlubar FF, Anderson KE. (2009) Phenotypic CYP2A6 variation and the risk of pancreatic cancer. JOP 10:263–270 [PMC free article] [PubMed] [Google Scholar]
- Kataoka H, Inoue R, Yagi K, Saito K. (2009) Determination of nicotine, cotinine, and related alkaloids in human urine and saliva by automated in-tube solid-phase microextraction coupled with liquid chromatography-mass spectrometry. J Pharm Biomed Anal 49:108–114 [DOI] [PubMed] [Google Scholar]
- Kedzierska K, Crowe SM. (2002) The role of monocytes and macrophages in the pathogenesis of HIV-1 infection. Curr Med Chem 9:1893–1903 [DOI] [PubMed] [Google Scholar]
- Levi M, Dempsey DA, Benowitz NL, Sheiner LB. (2007) Prediction methods for nicotine clearance using cotinine and 3-hydroxy-cotinine spot saliva samples II. Model application. J Pharmacokinet Pharmacodyn 34:23–34 [DOI] [PubMed] [Google Scholar]
- Manda VK, Mittapalli RK, Geldenhuys WJ, Lockman PR. (2010) Chronic exposure to nicotine and saquinavir decreases endothelial Notch-4 expression and disrupts blood-brain barrier integrity. J Neurochem 115:515–525 [DOI] [PubMed] [Google Scholar]
- Marclay F, Saugy M. (2010) Determination of nicotine and nicotine metabolites in urine by hydrophilic interaction chromatography-tandem mass spectrometry: Potential use of smokeless tobacco products by ice hockey players. J Chromatogr A 1217:7528–7538 [DOI] [PubMed] [Google Scholar]
- Miller EI, Norris HR, Rollins DE, Tiffany ST, Wilkins DG. (2010) A novel validated procedure for the determination of nicotine, eight nicotine metabolites and two minor tobacco alkaloids in human plasma or urine by solid-phase extraction coupled with liquid chromatography-electrospray ionization-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 878:725–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner LJ, Crowe SM, Aquaro S, Perno CF, Stevenson M, Collman RG. (2006) Advances in macrophage and dendritic cell biology in HIV-1 infection stress key understudied areas in infection, pathogenesis, and analysis of viral reservoirs. J Leukoc Biol 80:961–964 [DOI] [PubMed] [Google Scholar]
- Rock RB, Gekker G, Aravalli RN, Hu S, Sheng WS, Peterson PK. (2008) Potentiation of HIV-1 expression in microglial cells by nicotine: involvement of transforming growth factor-beta 1. J Neuroimmune Pharmacol 3:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakleya DM, Huestis MA. (2009) Simultaneous and sensitive measurement of nicotine, cotinine, trans-3′-hydroxycotinine and norcotinine in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 877:3537–3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu I, Wang P. (2013) Simultaneous serum nicotine, cotinine, and trans-3′-hydroxycotinine quantitation with minimal sample volume for tobacco exposure status of solid organ transplant patients. J Chromatogr B Analyt Technol Biomed Life Sci 928:139–145 [DOI] [PubMed] [Google Scholar]
- Vallari A, Bodelle P, Ngansop C, Makamche F, Ndembi N, Mbanya D, Kaptué L, Gürtler LG, McArthur CP, Devare SG, et al. (2010) Four new HIV-1 group N isolates from Cameroon: Prevalence continues to be low. AIDS Res Hum Retroviruses 26:109–115 [DOI] [PubMed] [Google Scholar]
- Wojna V, Robles L, Skolasky RL, Mayo R, Selnes O, de la Torre T, Maldonado E, Nath A, Meléndez LM, Lasalde-Dominicci J. (2007) Associations of cigarette smoking with viral immune and cognitive function in human immunodeficiency virus-seropositive women. J Neurovirol 13:561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi J, Bodelle P, Vallari AS, Coffey R, McArthur CP, Schochetman G, Devare SG, Brennan CA. (2004) HIV infections in northwestern Cameroon: identification of HIV type 1 group O and dual HIV type 1 group M and group O infections. AIDS Res Hum Retroviruses 20:944–957 [DOI] [PubMed] [Google Scholar]
- Yamaguchi J, McArthur CP, Vallari A, Coffey R, Bodelle P, Beyeme M, Schochetman G, Devare SG, Brennan CA. (2006) HIV-1 Group N: evidence of ongoing transmission in Cameroon. AIDS Res Hum Retroviruses 22:453–457 [DOI] [PubMed] [Google Scholar]
- Zhao L, Li F, Zhang Y, Elbourkadi N, Wang Z, Yu C, Taylor EW. (2010) Mechanisms and genes involved in enhancement of HIV infectivity by tobacco smoke. Toxicology 278:242–248 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.