Graphical abstract

Abstract
A chemoenzymatic strategy for the synthesis of enantiomerically pure novel alkaloids (1S,3R)-1-benzyl-2,3-dimethyl-1,2,3,4-tetrahydroisoquinolines is presented. The key steps are the biocatalytic stereoselective reductive amination of substituted 1-phenylpropan-2-one derivatives to yield chiral amines employing microbial ω-transaminases, and the diastereoselective reduction of a Bischler–Napieralski imine intermediate by catalytic hydrogenation in the presence of palladium on charcoal, leading exclusively to the desired cis-isomer.
1. Introduction
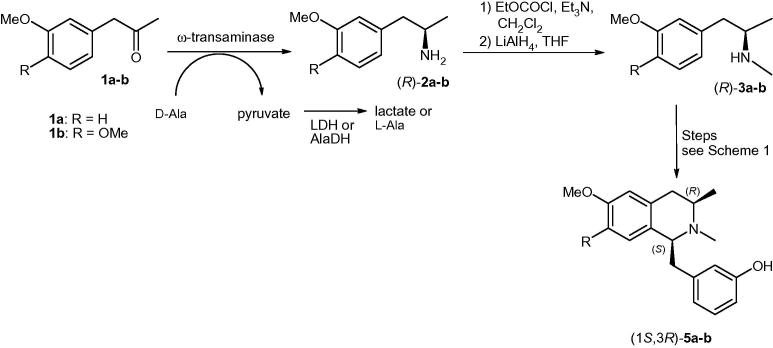
Various 1-benzyl-1,2,3,4-tetrahydroisoquinolines1 act as antispasmodic or hypotensive agents and some, such as norcoclaurine, coclaurine, and N-methylcoclaurine, possess anti-HIV activity in vitro.2 Consequently, benzylisoquinolines have been targets for organic synthesis for a long time, and their asymmetric synthesis has been achieved using many different strategies.3,4 Published synthetic routes involve metal-catalyzed asymmetric hydrogenations,5 intramolecular allylic aminations or amidations,6 photoinduced electrocyclic ring closure of aromatic enehydrazides,7 various metal- or organocatalyzed asymmetric alkylation reactions,8 or chemical Pictet–Spengler reactions.9 Biocatalytic approaches10 include deracemization employing an amine oxidase,11 kinetic resolution by the berberine bridge enzyme,12 or the enzymatic Pictet–Spengler reaction.9,13 Since in nature the (1S)-enantiomer is produced as a first metabolite by the norcoclaurine synthase,13 we set out to develop an alternative strategy to access substituted (1S)-1-benzyl-1,2,3,4-tetrahydroisoquinolines involving ω-transaminases (ω-TAs).14
2. Results and discussion
Our strategy was to control the stereochemistry at C1 of tetrahydroisoquinolines 5 by first introducing a stereocenter at C3, thus providing access to novel 1-benzyl-3-methyl-1,2,3,4-tetrahydroisoquinolines as final products. For the synthesis of chiral amines 2/3, ω-transaminases should be employed, while the ring closure could be performed chemically.
First, control of the relative configuration at position C1 in 5 by the stereogenic amine center of 2 was investigated under various reaction conditions (Scheme 1). For this purpose ketone 1 was aminated with methylamine to give racemic N-methyl amine 3, followed by amide formation with a phenylacetic acid chloride derivative. After the Bischler–Napieralski cyclization and further reduction with sodium borohydride in methanol, a mixture of the two diastereomers 5 was obtained in a trans:cis = 3:1 ratio. In contrast, the catalytic hydrogenation of the intermediate iminium ion of the Bischler–Napieralski reaction using palladium on charcoal in methanol led exclusively to the formation of the cis-isomer rac-cis-5. Additionally the protection groups were removed in the same reaction step, making this approach favorable.
Scheme 1.
Influence of the chemical reduction method on the relative stereochemistry of 5.
Since the cis-isomer 5 was obtained with perfect chemical stereocontrol, and given that (1S)-benzylisoquinolines were desired, the absolute configuration at the C3 position of 5 must be (R). Consequently, three different (R)-stereoselective ω-TAs were tested for the asymmetric amination of 1 to prepare the corresponding (R)-amine 2. The ω-TAs employed originate from Aspergillus terreus,15 Hyphomona neptunium,15 and Arthrobacter sp.16 d-Alanine was used as the amine donor for these enzymes, whereby the pyruvate formed was removed either with a lactate dehydrogenase (LDH) or an alanine dehydrogenase (AlaDH) to shift the equilibrium to the product side (Scheme 2).17 Furthermore, a variant of the Arthobacter sp. transaminase (ArRmut11-ωTA)18 was tested using 2-propylamine as the amine donor (Table 1). The best results were obtained at a substrate concentration of 50 mM employing lyophilized Escherichia coli cells containing the overexpressed ω-TAs from A. terreus and Arthrobacter sp. No significant difference was observed when pyruvate was removed by LDH or AlaDH.
Scheme 2.
Biocatalytic amination of ketone 1 followed by formal methylation leading to (1S,3R)-5a and 5b.
Table 1.
Asymmetric reductive amination of 1a (50 mM) employing ω-TAs from different microorganisms. AlaDH or LDH was used to remove the coproduct pyruvate
| ω-TA | Pyruvate removed by | Conv. a (%) | eeb (%) |
|---|---|---|---|
| A. terreus | LDHc | >99 | >99 (R) |
| A. terreus | AlaDH d | >99 | >99 (R) |
| Arthrobacter sp. | LDHc | >99 | >99 (R) |
| Arthrobacter sp. | AlaDH d | >99 | >99 (R) |
| H. neptunium | LDHc | 33 | >99 (R) |
| H. neptunium | AlaDH d | 37 | >99 (R) |
| ArRmut11-ω-TAe | n.a.f | 89 | >99 (R) |
The conversion after 24 h was determined by GC on an achiral phase and was calculated from the areas of the substrate and product peaks.
Determined by GC on a chiral phase of the samples derivatized as acetamides.
Reaction conditions: substrate (50 mM), d-alanine (250 mM), phosphate buffer (pH 7, 100 mM), PLP (1 mM), freeze-dried E. coli cells containing overexpressed ω-TA (20 mg), LDH (90 U), GDH (30 U), NAD+ (1 mM), glucose (150 mM).
Reaction conditions: substrate (50 mM), d-alanine (250 mM), phosphate buffer (pH 7, 100 mM), PLP (1 mM), freeze-dried E. coli cells containing overexpressed ω-TA (20 mg), AlaDH (12 U), FDH (11 U), NAD+ (1 mM), ammonium formate (150 mM).
Reaction conditions: substrate (50 mM), aqueous buffer (pH 11, 2-propylamine 1 M, PLP 0.5 mM), 20% v v−1 DMSO, E. coli cells containing overexpressed ω-TA (20 mg).
Not applicable. 2-Propylamine was used as the amine donor.
In order to improve the amination reaction, a twofold higher ketone concentration (100 mM) was tested as well as the influence of DMSO as a co-solvent (Table 2). The reaction was completed within 24 h only in the case of A. terreus transaminase in the absence of DMSO and using the AlaDH system.
Table 2.
Reductive amination of 1a (100 mM) after 24 h employing ω-transaminases (ω-TA) from A. terreus and Arthrobacter sp. LDH and AlaDH were used to remove pyruvate
| ω-TA | Pyruvate removed by | Conv. (%)a |
|
|---|---|---|---|
| DMSO 15% v/v | w/o DMSO | ||
| A. terreus | LDH systemb | 76 | 69 |
| A. terreus | AlaDHc | 93 | >99 |
| Arthrobacter sp. | LDH systemb | 72 | 66 |
| Arthrobacter sp. | AlaDHc | 75 | 71 |
In all cases, the ee was >99% as determined for the amine derivatized to the corresponding acetamides by GC on a chiral phase.
Reaction conditions: substrate (100 mM), d-alanine (500 mM), phosphate buffer (pH 7, 100 mM), PLP (1 mM), freeze-dried E. coli cells containing overexpressed ω-TA (20 mg), LDH (90 U), GDH (30 U), NAD+ (1 mM), glucose (300 mM).
Reaction conditions: substrate (100 mM), d-alanine (500 mM), phosphate buffer (pH 7, 100 mM), PLP (1 mM), freeze-dried E. coli cells containing overexpressed ω-TA (20 mg), AlaDH (12 U), FDH (11 U), NAD+ (1 mM), ammonium formate (300 mM).
For the total synthesis of enantiomerically pure (1S,3R)-5a and 5b, the reductive amination of the corresponding ketones was performed at 100 mM concentration and on a 50 mL scale (819/917 mg of ketones 1a–b, respectively). A. terreus ω-TA and the AlaDH system were used for the asymmetric amination of 1a–b leading to enantiomerically pure (R)-2a–b (ee >99%) in 92% and 77% isolated yield, respectively. Methylation of the primary amines12 yielded (R)-3a–b, which were condensed with 3-benzyloxyphenylacetyl chloride as shown in Scheme 1. Finally, 450 and 209 mg of (1S,3R)-5a and 5b were obtained, corresponding to an overall yield of 30% and 14%, respectively, over 6 steps.
3. Conclusion
By employing a Bischler–Napieralski cyclization followed by a reduction with Pd/C and hydrogen, the stereogenic center at C1 of benzylisoquinolines can be controlled using a chiral amine as the substrate. Thus, cis-1-benzyl-2,3-dimethylisoquinolines can be obtained in excellent enantiomeric and diastereomeric purity, making this method an effective procedure for the asymmetric synthesis of unnatural tetrahydroisoquinoline alkaloids bearing two stereogenic centers. The asymmetric key steps are the biocatalytic stereoselective amination of the prochiral ketones on one hand, as well as stereocontrolled reduction of the intermediate Bischler–Napieralski imines on the other hand.
4. Experimental
4.1. General
Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance 300 at operating frequencies of 300 MHz (1H NMR) or 75 MHz (13C NMR). Chemical shifts are given in parts per million (ppm) relative to TMS (δ = 0 ppm) and coupling constants (J) are reported in Hertz (Hz). Optical rotation values were measured at 589 nm (Na line) on a Perkin–Elmer Polarimeter 341 using a cuvette of 1 dm path length. Melting points were determined on a Gallenkamp MPD350 apparatus and are uncorrected. Thin layer chromatography was carried out on silica gel 60 F254 plates and compounds were visualized either by spraying with Mo reagent [(NH4)6Mo7O24·4H2O (100 g L−1), Ce(SO4)2·4H2O (4 g L−1) in H2SO4 (10%)] or by UV. Unit resolution GC–MS analyses were performed using electron impact (EI) ionization at 70 eV and quadrupole mass selection. High resolution MS analyses were performed using electron impact (EI) ionization at 70 eV and time-of-flight (TOF) mass selection. Unless otherwise noted, reagents and organic solvents were obtained from commercially available sources and were used without further purification. HPLC analysis was performed on a Shimadzu system (Communication Bus Module CBM-20 A, Column Oven CTO-20 AC, Degasser DGU-20 A5, Liquid Chromatograph LC-20 AD, Auto sampler SIL-20 AC, Diode Array Detector SPD-M20 A) using an achiral C18 column (Phenomenex, LUNA C18, 0.46 cm × 25 cm, 5 mm). Eluent: buffer (30 mM HCO2NH4, pH 2.8)/methanol/acetonitrile = 67/18/15 (isocratic); flow rate: 0.5 mL/min; column temperature: 20 °C; detection wavelength: 280 nm. HPLC of enantiomers was performed on a chiral stationary phase (Chiralcel OJ). Eluent: n-heptane/2-propanol = 90/10 + 0.1% formic acid (isocratic); flow rate: 0.75 mL/min.
GC analytics were performed using an Agilent 7890 A GC system, equipped with an FID detector and using an Agilent J&W DB-1701 column (30 m, 250 μm, 0.25 μm). Carrier: Helium at constant pressure: 1 bar. T injector: 250 °C. Program: 80 °C, hold 6.5 min. Gradient 10 °C/min up to 160 °C, hold 5 min. Gradient 20 °C/min up to 200 °C, hold 2 min. Retention times: 1a: 20.3 min, 2a: 19.3 min. 1b: 22.4 min, 2b: 21.6 min.
For the determination of the ee the column Varian Chrompack Chirasel Dex-CB (25 m, 320 μm, 0.25 μm) was employed. Carrier: Hydrogen at constant flow: 1.7 mL/min. T injector: 200 °C. Program: 100 °C, hold 2 min. Gradient 1 °C/min up to 130 °C, hold 5 min. Gradient: 10 °C/min up to 180 °C, hold 20 min. Retention times: (S)-2a: 44 min, (R)-2a: 44.3 min, (R)-2b: 47.6 min.
Enantiomeric purity of 5b: HPLC, Chiralcel OJ (Daicel Chemical Industries, 0.46 cm × 25 cm, Lot. No.: OJ00CE-NK006). Eluent: n-heptane (0.1% formic acid) and 2-propanol (0.1% formic acid) (70:30), flow rate 0.5 mL min−1 (isocratic), detection wavelength: 280 nm. Retention times: (1S,3R): 11.2 min, (1R,3S): 12.5 min.
Lyophilized E. coli cells containing overexpressed (R)-selective ω-TAs and AlaDH were prepared as previously described.17 l-Lactate dehydrogenase from rabbit muscle (lyophilized powder, 136 U mg−1 protein, one unit will reduce 1.0 μmol of pyruvate to l-lactate per min at pH 7 at 25 °C, catalog no. 61309) was purchased from Sigma–Aldrich (Vienna, Austria). Glucose dehydrogenase (lyophilized powder, 25 U mg−1, one unit will oxidize 1 μmol β-d-glucose to d-glucono-δ-lactone per min at pH 8.0 and 37 °C, catalog no. B-4) was purchased from X-zyme (Düsseldorf, Germany). Formate dehydrogenase from Candida boidinii (lyophilized crude enzyme, 2.2 U mg−1, one unit will oxidize 1.0 μmole of formate to CO2 per min at pH 7.6 at 37 °C, catalog no. FDH-101) and β-NAD free acid was purchased from Codexis (Redwood City, CA, USA).
4.2. Biocatalytic procedures
The biocatalytic reactions using ω-TAs were performed at 30 °C in a sodium phosphate buffer (100 mM, pH 7) containing pyridoxal 5′-phosphate (PLP, 1 mM) and NAD+ (free acid, 1 mM). DMSO was added in the indicated experiments (15%, v v−1). Biotransformations with ArRmut11-ωTA were performed at 45 °C in an aqueous solution of 2-propylamine (1 M), at pH 11 (adjusted with aqueous 6 M HCl) in the presence of DMSO (20%, v v−1) as cosolvent and pyridoxal 5′-phosphate (0.5 mM). Eppendorf tubes were shaken on orbital shakers in a horizontal position to ensure improved mixing.
4.2.1. Representative example for the reductive amination employing the AlaDH system
Lyophilized cells of E. coli BL21 (DE3) containing overexpressed ω-TA (20 mg) were rehydrated in an Eppendorf tube (2 mL) in a phosphate buffer (1 mL, 100 mM, pH 7, 1 mM PLP, 1 mM NAD+) for 30 min at 30 °C and 120 rpm on an orbital shaker. Ammonium formate (150 mM), d-alanine (250 mM, 22.3 mg), l-alanine dehydrogenase (12 U), or disrupted cells of E. coli BL21 (DE3) containing overexpressed AlaDH (20 mg), formate dehydrogenase (11 U), and ketone 1a (50 mM) were added. In the experiments with 100 mM substrate concentration, the amounts of d-alanine and the ammonium formate were doubled (500 mM and 300 mM, respectively). DMSO was also added in selected experiments (15%, v v−1). The mixture was shaken at 30 °C and 120 rpm. The reaction was quenched after 24 h by the addition of aqueous 10 M NaOH (200 μL) and the reaction mixture was extracted with ethyl acetate (2 × 500 μL). The combined organic phases were dried (Na2SO4) and the conversion was measured by GC.
4.2.2. Representative example for the amination employing the LDH system
Lyophilized cells of E. coli BL21 (DE3) containing overexpressed ω-TA (20 mg) were rehydrated in an Eppendorf tube (2 mL) in phosphate buffer (1 mL, 100 mM, pH 7, 1 mM PLP, 1 mM NAD+) for 30 min at 30 °C and 120 rpm on an orbital shaker. Glucose (150 mM), d-alanine (250 mM, 22.3 mg), lactate dehydrogenase (90 U), glucose dehydrogenase (30 U), and ketone 1a (50 mM) were added. DMSO was also added in selected experiments (15% v v−1). The mixture was shaken at 30 °C and 120 rpm. The reaction was quenched and worked up as described above.
4.2.3. Representative example for amination employing the 2-propylamine system
Lyophilized cells of E. coli BL21 (DE3)/pET21a-ArRmut11-ω-TA (20 mg) were rehydrated in a glass vial (4 mL) in an aqueous buffer (800 μL, pH 11, 0.5 mM PLP) containing 2-propylamine (1 mmol) for 30 min at 30 °C and 120 rpm on an orbital shaker. Then, DMSO (200 μL) and ketone 1a (50 mM) were added. The vial was closed with an airtight cap and shaken at 45 °C and 120 rpm in an upright position. The reaction was quenched and worked up as described above.
4.2.4. Up-scaling of the amination of ketones 1a and 1b
Lyophilized cells of E. coli BL21 (DE3) containing overexpressed AlaDH from Bacillus subtilis (0.5 g) were suspended in a phosphate buffer (50 mL, 100 mM, pH 7) and subjected to ultrasonication with a Branson Digital Sonifier (8 min, 40% Amplitude, 1 s Pulse, 4 s Pause). The disrupted cells were centrifuged (16,000 rpm, 15 min, 4 °C) and the supernatant separated and divided into two aliquots in conical bottom centrifuge tubes with screw caps (25 mL/tube). Lyophilized cells of E. coli BL21 (DE3) containing overexpressed ω-transaminase from A. terreus were added to each tube (0.5 g) and rehydrated for 30 min at 30 °C and 120 rpm on an orbital shaker. Then, PLP (1 mM, 6.63 mg), NAD+ (1 mM, 17 mg), ammonium formate (300 mM, 480 mg), d-alanine (500 mM, 1115 mg), formate dehydrogenase (11 U, 125 mg) and ketones 1a or 1b (100 mM) were added. The mixture was shaken at 30 °C and 120 rpm. The reaction was quenched after 24 h by the addition of aqueous 10 M NaOH (pH 10) and the reaction mixture was extracted with EtOAc (3 × 30 mL). The combined organic phases were dried (Na2SO4) and conversions were determined by GC as described above.
1-(3-Methoxyphenyl)propan-2-amine (R)-2a
Obtained as a pale yellow liquid. Yield: 763 mg (92%). ee: >99%. (c 1.13, CHCl3). Lit.19 for (S)-2a (c 1.00 CHCl3) ee: 96%. 1H NMR (CDCl3, 300 MHz): δ = 1.14 (3H, d, J = 6.3 Hz, CH3), 1.49 (2H, br s, NH2), 2.50 (1H, dd, J = 8.1, 13.3 Hz, CH2CH), 2.71 (1H, dd, J = 5.4, 13.3 Hz, CH2CH), 3.22–3.15 (1H, m, CH2CH), 3.81 (3H, s, OCH3), 6.80–6.75 (3H, m, Ar), 7.23 (1H, t, J = 7.6 Hz, Ar). 13C NMR (CDCl3, 75 MHz): δ = 23.6, 46.7, 48.4, 55.1, 111.5, 115.0, 121.6, 129.4, 141.3, 159.6. HRMS calculated for C10H15NO [M+]: 165.1154; found: 165.1159.
1-(3,4-Dimethoxyphenyl)propan-2-amine (R)-2b
Obtained as a viscous liquid which formed white crystals after prolonged standing. Yield: 711 mg (77%). Mp: 104–105 °C. ee: >99%. (c 1.00, CHCl3). Lit.20 for (R)-2b (c 4.13, CHCl3). 1H NMR (CDCl3, 300 MHz): δ = 1.12 (3H, d, J = 6.3 Hz, CH3), 1.49 (2H, br s, NH2), 2.44 (1H, dd, J = 8.1, 13.4 Hz, CH2CH), 2.67 (1H, dd, J = 5.1, 13.4 Hz, CH2CH), 3.18–3.11 (1H, m, CH2CH), 3.86 (3H, s, OCH3), 3.87 (3H, s, OCH3), 6.74–6.71 (2H, m, Ar), 6.81 (1H, d, J = 8.1 Hz, Ar). 13C NMR (CDCl3, 75 MHz): δ = 23.5, 46.1, 48.5, 55.8, 55.9, 111.2, 112.3, 121.1, 132.3, 147.4, 148.8. HRMS calculated for C11H17NO2 [M+]: 195.1259; found: 195.1266.
4.3. Determination of the enantiomeric purity
The enantiomeric excess (ee) of amines 2a and 2b was analyzed by GC on a chiral phase after derivatization to the corresponding acetamides. Derivatization was performed by adding 4-(N,N-dimethylamino)pyridine (DMAP, 5 mg) dissolved in acetic anhydride (100 mL). After washing with water and drying (Na2SO4), the ee value of the derivatized compound was measured by GC.
4.4. General procedure for the preparation of rac-3a–b
The carbonyl compounds 1a–b (10 mmol), titanium(IV) isopropoxide (5.9 mL, 20 mmol), methylamine hydrochloride (1.35 g, 20 mmol), and triethylamine (2.79 mL, 20 mmol) in absolute EtOH (15 mL) were stirred under an argon atmosphere at ambient temperature for 16 h. Sodium borohydride (0.57 g, 15 mmol) was then added and the resulting mixture was stirred for an additional 6 h at ambient temperature. The reaction was then quenched by pouring into NH4OH (2 M, 30 mL). The resulting inorganic precipitate was filtered off and washed with CH2Cl2 (50 mL). The organic layer was separated and the remaining aqueous layer was extracted once with CH2Cl2 (50 mL). The combined organic layers were then extracted once with HCl (1 M, 25 mL) to separate the neutral materials. The acidic aqueous layer was washed once with CH2Cl2 (50 mL), then basified with aqueous NaOH (2 M) to pH 10–12 and extracted with CH2Cl2 (50 mL × 3). The combined organic extracts were washed with brine (50 mL), dried (Na2SO4), and concentrated in vacuo to afford the N-methyl secondary amines.
1-(3-Methoxyphenyl)-N-methylpropane-2-amine rac-3a
Obtained as a yellow oil. Isolated yield 1.29 g (72%). 1H NMR (CDCl3, 300 MHz) δ = 1.08 (3H, d, J = 6.3 Hz, CH3), 2.05 (1H, br s, NH), 2.41 (3H, s, NCH3), 2.85–2.58 (3H, m overlap, CH2-CH), 3.81 (3H, s, OCH3), 6.80–6.75 (3H, m, Ar), 7.22 (1H, t, J = 7.7 Hz, Ar). 13C NMR (CDCl3, 75 MHz): δ = 19.8, 34.0, 43.6, 55.0, 56.2, 111.4, 115.0, 121.7, 129.3, 141.1, 159.6. HRMS calculated for C11H17NO [M+−H]: 178.1232; found: 178.1225.
1-(3,4-Dimethoxyphenyl)-N-methylpropan-2-amine rac-3b
Obtained as a viscous yellow oil. Isolated yield 1.34 g (64%). 1H NMR (CDCl3, 300 Mhz) δ = 1.04 (3H, d, J = 6.0 Hz, CH3), 1.49 (1H, br s, NH), 2.37 (3H, s, NCH3), 2.58 (2H, d, J = 5.8 Hz, CH2-CH), 2.73 (1H, m, CH2-CH), 3.85 (3H, s, OCH3), 3.86 (3H, s, OCH3), 6.80–6.70 (3H, m, Ar). 13C NMR (CDCl3, 75 MHz): δ = 19.8, 34.2, 43.3, 55.9, 56.5, 111.3, 112.5, 121.3, 132.1, 147.5, 148.9. HRMS calculated for C12H19NO2: [M+−H]: 208.1338; found: 208.1348.
4.5. General procedure for the preparation of rac-4a–b
Amines 3a–b (10 mmol of 3a or 6.4 mmol of 3b) were dissolved in CHCl3 (30 mL). Next, 3% aqueous NaOH solution (150 mL) was added and the mixture was cooled to 0 °C using an ice bath. A solution of 3-benzyloxyphenylacetyl chloride (2.88 g, 11.0 mmol) in chloroform (20 mL) was added dropwise over 1 h to the vigorously stirred mixture. The ice bath was removed and stirring was continued for 2 h at room temperature. The phases were separated and the aqueous layer was extracted with CHCl3 (50 mL). The combined organic phases were washed with dilute HCl solution (0.1 N, 100 mL), then brine (100 mL), and dried over Na2SO4. Evaporation of the solvent under reduced pressure yielded a highly viscous yellow liquid. Flash chromatography (silica; petrol ether/EtOAc = 6.6/3.5) afforded the corresponding methylacetamides.
2-(3-(Benzyloxy)phenyl)-N-(1-(3-methoxyphenyl)propan-2-yl)-N-methylacetamide rac-4a
Obtained as a pale yellowish liquid (2.99 g, 74%). The NMR spectra revealed the presence of a mixture of the cis- and trans-isomers. 1H NMR (CDCl3, 300 MHz): δ = 0.95 (3H, d, J = 6.6 Hz, CH-CH3), 1.02 (3H, d, J = 6.9 Hz, CH-CH3), 2.53–2.61 (2H, m, Ph-CH2-CH), 2.63–2.66 (2H, m, Ph-CH2-CH), 2.68 (3H, s, N-CH3), 2.79 (3H, s, N-CH3), 3.32 (2H, s, Ph-CH2-CO), 3.52 (2H, s, Ph-CH2-CO), 3.67 (6H, s, OCH3), 3.94–3.99 (1H, m, CH), 4.92 (2H, s, Ph-CH2-O), 4.95 (2H, s, Ph-CH2-O), 4.99–5.03 (1H, m, CH), 6.46–6.77 (12H, m, Ar), 7.05–7.36 (14H, m, Ar). 13C NMR (CDCl3, 75 MHz): δ = 17.2, 18.4, 26.6, 29.5, 40.1, 40.7, 41.2, 41.8, 49.4, 53.4, 54.7, 55.2, 69.4, 69.9, 111.8, 112.0, 113.1, 113.3, 114.3, 114.8, 115.0, 115.1, 121.2, 121.3, 127.4, 127.5, 127.9, 128.5, 129.3, 129.6, 136.6, 136.9, 137.1, 139.9, 140.0, 159.0, 159.6, 159.8, 170.6, 170.7. HRMS calculated for C26H29NO3 [M+]: 403.2148; found: 403.2151.
2-(3-(Benzyloxy)phenyl)-N-(1-(3,4-dimethoxyphenyl)propan-2-yl)-N-methylacetamide rac-4b
Obtained as a brownish liquid (1.60 g, 58%). The NMR spectra revealed the presence of a mixture of the cis- and trans-isomers. 1H NMR (CDCl3, 300 MHz): δ = 1.05 (3H, d, J = 6.5 Hz, CH-CH3), 1.11 (3H, d, J = 6.7 Hz, CH-CH3), 2.56–2.62 (2H, m, Ph-CH2-CH), 2.68–2.70 (2H, m, Ph-CH2-CH), 2.75 (3H, s, N-CH3), 2.89 (3H, s, N-CH3), 3.39 (2H, s, Ph-CH2-CO), 3.60 (2H, s, Ph-CH2-CO), 3.83 (6H, s, OCH3), 3.84 (6H, s, OCH3), 4.03–4.00 (1H, m, CH), 5.04 (2H, s, Ph-CH2-O), 5.08 (2H, s, Ph-CH2-O), 5.11–5.08 (1H, m, CH), 6.86–6.49 (12H, m, Ar), 7.44–7.14 (12H, m, Ar). 13C NMR (CDCl3, 75 MHz): δ = 17.2, 18.4, 26.7, 29.5, 39.7, 40.3, 41.3, 41.9, 49.3, 55.0, 55.8, 55.9, 56.0, 69.8, 69.9, 111.0, 111.4, 112.0, 112.1, 113.1, 115.0, 115.2, 120.9, 121.0, 121.1, 121.2, 127.5, 127.6, 128.0, 128.6, 129.7, 130.9, 131.0, 136.6, 137.0, 137.1, 147.5, 147.8, 148.8, 149.0, 159.0, 159.1, 170.7, 170.8. HRMS calculated for C27H31NO4 [M+]: 433.2253; found: 433.2254.
4.6. General procedure for the preparation of rac-5a–b
To a solution of 4a–b (7.18 mmol of 4a or 4.32 mmol of 4b) in dry acetonitrile (70 mL), POCl3 (3.23 g, 21.1 mmol) was added and the mixture was refluxed for 3 h under an argon atmosphere. The solvent and excess POCl3 were evaporated under reduced pressure and the residue was dissolved in dry methanol, put under argon and cooled to 0 °C using an ice bath. Next, NaBH4 (1.98 g, 52.2 mmol) was added in portions to the stirred mixture. The ice bath was then removed and stirring was continued for 14 h at room temperature. The solvent was evaporated and the residue was treated with a half-saturated Na2CO3 solution (100 mL). The product was extracted with CH2Cl2 (3 × 50 mL), the combined organic phases were dried over Na2SO4 and evaporated under reduced pressure to give a pale yellowish liquid. Analysis by TLC showed two products, which were separated by flash chromatography (silica; EtOAc/MeOH = 95/5). Each product was treated with Pd (10% on activated charcoal; 200 mg), acetic acid (470 mg, 7.8 mmol), and dry methanol (20 mL) and stirred under an H2 atmosphere (balloon) for 16 h. The mixture was filtered through Celite, washed with methanol (50 mL), and evaporated under reduced pressure. The residue was dissolved in CH2Cl2 and washed with a half-saturated NaHCO3 solution (40 mL). The organic phase was dried over Na2SO4 and evaporated under reduced pressure. The residue was purified by silica gel flash chromatography (EtOAc/MeOH = 95/5).
cis-1-(3-Hydroxybenzyl)-6-methoxy-2,3-dimethyl-1,2,3,4-tetrahydroisoquinoline cis-5a
Obtained as white crystals, which were not soluble in CH2Cl2 or CHCl3. Yield 298 mg (14%). Mp: 125–126 °C. 1H NMR (MeOD, 300 MHz): δ = 1.24 (3H, d, J = 5.7 Hz, CH3-CH), 2.41 (3H, s, NCH3), 2.52–2.58 (3H, m overlap, Ph-CH2-CH-CH3), 2.83 (1H, dd, J = 7.5, 13.8 Hz, Ph-CH2-CH), 3.06 (1H, dd, J = 4.5, 13.8 Hz, Ph-CH2-CH), 3.73 (3H, s, OCH3), 3.83 (1H, dd, J = 4.5, 7.5 Hz, Ph-CH2-CH), 6.57–6.62 (5H, m, Ar), 6.71–6.75 (1H, m, Ar), 7.01–7.06 (1H, m, Ar). 13C NMR (MeOD, 75 MHz): δ = 20.0, 36.5, 38.6, 43.7, 54.1, 55.7, 66.7, 111.3, 111.9, 112.5, 116.2, 120.6, 127.8, 128.5, 129.5, 136.7, 141.2, 156.7, 158.0. HRMS calculated for C19H23NO2 [M+−H]: 296.1650; found: 296.1664. Retention time on HPLC (achiral C18 column, details see above): 26.2 min.
trans-1-(3-Hydroxybenzyl)-6-methoxy-2,3-dimethyl-1,2,3,4-tetrahydroisoquinoline trans-5a
Obtained as a yellow solid foam. Yield 946 mg (44%). Mp: 46–49 °C. 1H NMR (CDCl3, 300 MHz): δ = 1.28 (3H, d, J = 6.6 Hz, CH3-CH), 2.39 (3H, s, NCH3), 2.66–2.70 (2H, m, Ph-CH2-CH), 2.82 (1H, dd, J = 8.4, 13.2 Hz Ph-CH2-CH), 3.25 (1H, dd, J = 4.8, 13.2 Hz Ph-CH2-CH), 3.46–3.54 (1H, m, CH-CH3), 3.76 (3H, s, OCH3), 3.94 (1H, dd, J = 4.8, 8.4 Hz, Ph-CH2-CH), 6.46–6.62 (4H, m, Ar), 4.70–6.72 (2H, m, Ar), 7.07–7.13 (1H, m, Ar). 13C NMR (CDCl3, 75 MHz): δ = 18.7, 31.8, 35.7, 42.2, 47.3, 55.1, 55.2, 66.0, 111.6, 113.2, 113.8, 116.3, 121.8, 128.4, 129.2, 129.3, 134.8, 141.2, 156.6, 157.9. HRMS calculated for C19H23NO2 [M+−H]: 296.1650; found: 296.1646. Retention time on HPLC (achiral C18 column, details see above): 25.9 min.
cis-1-(3-Hydroxybenzyl)-6,7-dimethoxy-2,3-dimethyl-1,2,3,4-tetrahydroisoquinoline cis-5b
Obtained as white crystals, which were not soluble in CH2Cl2 or CHCl3. Yield 246 mg (17%). Mp: 159–161 °C. 1H NMR (CDCl3, 300 MHz, analyzed as a benzyloxy derivative) δ = 1.22 (3H, d, J = 5.7 Hz, CH3-CH), 2.46 (3H, s, NCH3), 2.55–2.48 (3H, m overlap Ph-CH2-CH-CH3), 2.89–2.75 (1H, m, Ph-CH2-CH), 3.14 (1H, dd, J = 3.3, 14.5 Hz, Ph-CH2-CH), 3.58 (3H, s, OCH3), 3.78–3.75 (1H, m, Ph-CH2-CH), 3.83 (3H, s, OCH3) 4.99 (2H, s, Ph-CH2-O), 6.20 (1H, s, Ar), 6.53 (1H, s, Ar), 6.82–6.69 (3H, m, Ar). 7.14 (1H, t, J = 7.8 Hz, Ar), 7.44–7.30 (5H, m, Ar). 13C NMR (CHCl3, 75 MHz): δ = 21.7, 37.1, 40.4, 44.9, 55.4, 55.8, 67.1, 70.0, 76.7, 77.1, 77.6, 110.4, 112.4, 116.6, 122.9, 127.5, 127.9, 128.1, 128.6, 128.9, 129.6, 137.2, 141.6, 146.6, 147.0, 158.5. HRMS calculated for C20H25NO3 [M+−H]: 326.1756; found: 326.1735.
trans-1-(3-Hydroxybenzyl)-6,7-dimethoxy-2,3-dimethyl-1,2,3,4-tetrahydroisoquinoline trans-5b
Obtained as yellow crystals. Yield 703 mg (49%). Mp: 82–84 °C. 1H NMR (CDCl3, 300 MHz) δ = 1.76 (3H, d, J = 6.0 Hz, CH3-CH), 2.99 (3H, s, NCH3), 3.16–3.07 (2H, m, Ph-CH2-CH-CH3), 3.34–3.22 (1H, m, Ph-CH2-CH), 3.77–3.69 (1H, m, Ph-CH2-CH), 3.83 (3H, s, OCH3) 4.52–4.36 (4H, overlap s + m, OCH3, Ph-CH2-CH-CH3), 4.57–4.54 (1H, m, Ph-CH2-CH), 6.25 (1H, s, Ar), 6.95 (1H, s, Ar), 7.13–7.03 (3H, m, Ar), 7.58–7.53 (1H, m, Ar). 13C NMR (CHCl3, 75 MHz): δ = 19.2, 31.5, 36.1, 42.1, 47.3, 55.4, 55.8, 66.6, 111.1, 112.4, 116.5, 122.7, 125.8, 142.0, 146.3, 147.3, 158.7. HRMS calculated for C20H25NO3 [M+−H]: 326.1756; found: 326.1724.
4.7. General procedure for the preparation of rac-cis-5a–b
To a solution of 4a–b (50 mg, 0.12 mmol) in dry acetonitrile (3 mL), POCl3 (56 mg, 0.36 mmol) was added and the mixture was refluxed for 3 h under an argon atmosphere. The solvent and excess POCl3 were evaporated under reduced pressure and the residue was dissolved in dry methanol (3 mL). Next, Pd (10% on activated charcoal; 30 mg) was added and the mixture was stirred under an H2 atmosphere (balloon) for 5 h. The solids were removed by filtration through Celite, washed with MeOH (10 mL), and the solvent was evaporated under reduced pressure. The residue was dissolved in CH2Cl2 and washed with a half-saturated NaHCO3 solution (15 mL). The organic phase was dried over Na2SO4 and evaporated under reduced pressure. The products of the reaction were analyzed by GC–MS and TLC (EtOAc/MeOH = 95/5) and their Rf and tr compared to cis-5a and cis-5b obtained as described above.
4.8. Preparation of (R)-3a–b
To a solution of (R)-2a (760 mg, 4.6 mmol) in CH2Cl2 (20 mL) were added triethylamine (512 mg, 5.06 mmol) and ethyl chloroformate (599 mg, 5.52 mmol) and the mixture was stirred for 3 h at room temperature. Water (25 mL) was added, the phases were separated, and the aqueous phase was extracted with CH2Cl2 (2 × 15 mL). The combined organic phases were dried over Na2SO4 and evaporated under reduced pressure to give the corresponding carbamates.
(R)-Ethyl 1-(3-methoxyphenyl)propan-2-ylcarbamate
Compound was obtained in 1007 mg (92%) as a yellow liquid. (c 0.83, CHCl3).
(R)-Ethyl 1-(3,4-dimethoxyphenyl)propan-2-ylcarbamate
Compound was obtained in 773 mg (86%) as a white solid (mp: 81–83 °C). (c 1.00, CHCl3).
A solution of the corresponding carbamates (2.5 mmol) in anhydrous THF (15 mL) under argon atmosphere was cooled to 0 °C using an ice bath. LiAlH4 (805 mg, 21 mmol) was added in portions to the stirred solution; afterward the ice bath was removed and the mixture was refluxed for 6 h. The suspension was diluted with THF (15 mL) and cooled to 0 °C on an ice bath. To the vigorously stirred mixture were added: water (805 μL), 15% NaOH solution (805 μL), and again water (2.415 mL). Then, the ice bath was removed and stirring continued for 1 h at room temperature. The resulting suspension was filtered through Celite, washed with THF, dried over Na2SO4, and evaporated under reduced pressure. Purification was performed by flash chromatography (silica; CH2Cl2/MeOH/NH4OH = 90/9/1).
(R)-1-(3-methoxyphenyl)-N-methylpropan-2-amine (R)-3a
Compound was obtained in 534 mg (70%) as a yellow liquid. (c 2.35, CH2Cl2). Lit.21 for (S)-3a (c 1.98, CH2Cl2).
(R)-1-(3,4-dimethoxyphenyl)-N-methylpropan-2-amine (R)-3b
Compound was obtained in 297 mg (57%) as a yellow liquid.
(1S,3R)-5a
This compound was prepared following the procedure as described for rac-5a–b. Yield: 450 mg (30% over 6 steps from 1a). (c 1.79, MeOH).
(1S,3R)-5b
This compound was prepared following the procedure as described for rac-5a–b. Yield: 209 mg (14% over 6 steps from 1b). (c 0.18, MeOH).
Acknowledgments
Erasmus Mundus External Action 2 Programme is acknowledged for the financial support granted to A.A.O. We thank Professor Dr. Klaus Zangger for NMR analysis. F.G.M. received funding from the European Union’s Seventh Framework Programme FP7/2007–2013 under grant agreement n° 245144 (AmBioCas). J.H.S. and V.R. were financed by the Austrian Science Fund (FWF Project P20903-N17 and P22115-N17).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Alejandro A. Orden, Email: ale_orden@yahoo.com.ar.
Wolfgang Kroutil, Email: wolfgang.kroutil@uni-graz.at.
References
- 1.Bentley K.W. Harwood Academic Publishers; Amsterdam: 1998. The Isoquinoline Alkaloids. [Google Scholar]
- 2.(a) Martin M.L., Diaz M.T., Montero M.J., Prieto P., Roman L.S., Cortes D. Planta Med. 1993;59:63–67. doi: 10.1055/s-2006-959606. [DOI] [PubMed] [Google Scholar]; (b) Chulia S., Ivorra M.D., Lugnier C., Vila E., Noguera M.A., D’Ocon P. Br. J. Pharmacol. 1994;113:1377–1385. doi: 10.1111/j.1476-5381.1994.tb17150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kashiwada Y., Aoshima A., Ikeshiro Y., Chen Y.-P., Furukawa H., Itoigawa M., Fujioka T., Mihashi K., Cosentino L.M., Morris-Natschke S.L., Lee K.-H. Bioorg. Med. Chem. 2005;13:443–448. doi: 10.1016/j.bmc.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Chrzanowska M., Rozwadowska M.D. Chem. Rev. 2004;104:3341–3370. doi: 10.1021/cr030692k. For a review see: [DOI] [PubMed] [Google Scholar]
- 4.(a) Meyers A.I. Tetrahedron. 1992;48:2589–2612. [Google Scholar]; (b) Meyers A.I., Nguyen T.H. Heterocycles. 1994;39:513–518. [Google Scholar]; (c) Matulenko M.A., Meyers A.I. J. Org. Chem. 1996;61:573–580. doi: 10.1021/jo951611q. [DOI] [PubMed] [Google Scholar]
- 5.(a) Noyori R., Ohta M., Hsiao Y., Kitamura M., Ohta T., Takaya H. J. Am. Soc. Chem. 1986;108:7117–7119. [Google Scholar]; (b) Kitamura M., Hsiao Y., Ohta M., Tsukamoto M., Ohta T., Takaya H., Noyori R. J. Org. Chem. 1994;59:297–310. [Google Scholar]; (c) Mujahidin D., Doye S. Eur. J. Org Chem. 2005:2689–2693. [Google Scholar]; (d) Lu S.-M., Wang Y.-Q., Han X.-W., Zhou Y.-G. Angew. Chem., Int. Ed. 2006;45:2260–2263. doi: 10.1002/anie.200503073. [DOI] [PubMed] [Google Scholar]; (e) Yan P.-C., Xie J.-H., Hou G.-H., Wang L.-X., Zhou Q.-L. Adv. Synth. Catal. 2009;351:3243–3250. [Google Scholar]
- 6.(a) Ito K., Akashi S., Saito B., Katsuki T. Synlett. 2003:1809–1812. [Google Scholar]; (b) Shi C., Ojima I. Tetrahedron. 2007;63:8563–8570. [Google Scholar]; (c) Teichert J.F., Fañanás-Mastral M., Feringa B.L. Angew. Chem., Int. Ed. 2011;50:688–691. doi: 10.1002/anie.201006039. [DOI] [PubMed] [Google Scholar]
- 7.Dubois M., Deniau E., Couture A., Grandclaudon P. Tetrahedron. 2012;68:7140–7147. [Google Scholar]
- 8.(a) Ukaji Y., Shimizu Y., Kenmoku Y., Ahmed A., Inomata K. Bull. Chem. Soc. Jpn. 2000;73:447–452. [Google Scholar]; (b) Sasamoto N., Dubs C., Hamashima Y., Sodeoka M. J. Am. Chem. Soc. 2006;128:14010–14011. doi: 10.1021/ja065646r. [DOI] [PubMed] [Google Scholar]; (c) Dubs C., Hamashima Y., Sasamoto N., Seidel T.M., Suzuki S., Hashizume D., Sodeoka M. J. Org. Chem. 2008;73:5859–5871. doi: 10.1021/jo800800y. [DOI] [PubMed] [Google Scholar]; (d) Taylor A.M., Schreiber S.L. Org. Lett. 2006;8:143–146. doi: 10.1021/ol0526165. [DOI] [PubMed] [Google Scholar]; (e) Itoh T., Miyazaki M., Fukuoka H., Nagata K., Ohsawa A. Org. Lett. 2006;8:1295–1297. doi: 10.1021/ol0530326. [DOI] [PubMed] [Google Scholar]; (f) Wang S., Seto C.T. Org. Lett. 2006;8:3979–3982. doi: 10.1021/ol0614525. [DOI] [PubMed] [Google Scholar]; (g) Li Z., MacLeod P.D., Li C.-J. Tetrahedron: Asymmetry. 2006;17:590–597. [Google Scholar]; (h) Kanemitsu T., Yamashita Y., Nagata K., Itoh T. Synlett. 2006:1595–1597. [Google Scholar]
- 9.Stöckigt J., Antonchick A.P., Wu F., Waldmann H. Angew. Chem., Int. Ed. 2011;50:8538–8564. doi: 10.1002/anie.201008071. [DOI] [PubMed] [Google Scholar]
- 10.(a) Müller M. Adv. Synth. Catal. 2012;354:3161–3174. [Google Scholar]; (b) Resch V., Schrittwieser J.H., Siirola E., Kroutil W. Curr. Opin. Biotechnol. 2011;22:793–799. doi: 10.1016/j.copbio.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hammer S.C., Dominicus J.M., Syrén P.-O., Nestl B.M., Hauer B. Tetrahedron. 2012;68:7624–7629. [Google Scholar]
- 11.(a) Bailey K.R., Ellis A.J., Reiss R., Snape T.J., Turner N.J. Chem. Commun. 2007:3640–3642. doi: 10.1039/b710456a. [DOI] [PubMed] [Google Scholar]; (b) Foulkes J.M., Malone K.J., Coker V.S., Turner N.J., Lloyd J.R. ACS Catal. 2011;1:1589–1594. [Google Scholar]; (c) Rowles I., Malone K.J., Etchells L.L., Willies S.C., Turner N.J. ChemCatChem. 2012;4:1259–1261. [Google Scholar]
- 12.(a) Schrittwieser J.H., Resch V., Sattler J.H., Lienhart W.-D., Winkler A., Gruber K., Macheroux P., Kroutil W. Angew. Chem., Int. Ed. 2011;50:1068–1071. doi: 10.1002/anie.201006268. [DOI] [PubMed] [Google Scholar]; (b) Resch V., Schrittwieser J.H., Wallner S., Macheroux P., Kroutil W. Adv. Synth. Catal. 2011;13:2377–2383. [Google Scholar]; (c) Schrittwieser J.H., Resch V., Wallner S., Lienhart W.-D., Sattler J.H., Resch J., Macheroux P., Kroutil W. J. Org. Chem. 2011;76:6703–6714. doi: 10.1021/jo201056f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Resch V., Lechner H., Schrittwieser J.H., Wallner S., Gruber K., Macheroux P., Kroutil W. Chem. Eur. J. 2012;18:13173–13179. doi: 10.1002/chem.201201895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Ruff B.M., Bräse S., O’Connor S.E. Tetrahedron Lett. 2012;53:1071–1074. doi: 10.1016/j.tetlet.2011.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Pesnot T., Gershater M.C., Ward J.M., Hailes H.C. Adv. Synth. Catal. 2012;354:2997–3008. [Google Scholar]
- 14.(a) Malik M.S., Park E.-S., Shin J.-S. Appl. Microbiol. Biotechnol. 2012;94:1163–1171. doi: 10.1007/s00253-012-4103-3. For recent reviews on ω-transaminases see: [DOI] [PubMed] [Google Scholar]; (b) Mathew S., Yun H. ACS Catal. 2012;2:993–1001. [Google Scholar]; (c) Tufvesson P., Lima-Ramos J., Jensen J.S., Al-Haque N., Neto W., Woodley J.M. Biotechnol. Bioeng. 2011;108:1479–1493. doi: 10.1002/bit.23154. [DOI] [PubMed] [Google Scholar]; (d) Koszelewski D., Tauber K., Faber K., Kroutil W. Trends Biotechnol. 2010;28:324–332. doi: 10.1016/j.tibtech.2010.03.003. [DOI] [PubMed] [Google Scholar]; (e) Turner N.J., Truppo M. In: Chiral Amine Synthesis: Methods, Developments and Applications. Nugent T.C., editor. Wiley-VCH; Weinheim: 2010. pp. 431–459. [Google Scholar]; (f) Hailes H.C., Dalby P.A., Lye G.J., Baganz F., Micheletti M., Szita N., Ward J.M. Curr. Org. Chem. 2010;14:1883–1893. [Google Scholar]; (g) Ward J., Wohlgemuth R. Curr. Org. Chem. 2010;14:1914–1927. [Google Scholar]; (h) Höhne M., Bornscheuer U.T. ChemCatChem. 2009;1:42–51. [Google Scholar]
- 15.Höhne M., Schätzle S., Jochens H., Robins K., Bornscheuer U.T. Nat. Chem. Biol. 2010;6:807–813. doi: 10.1038/nchembio.447. [DOI] [PubMed] [Google Scholar]
- 16.Yamada, Y.; Iwasaki, A.; Kizaki N. (Kaneka Corporation), 2000, EP 0987332 A1.
- 17.Mutti F.G., Fuchs C.S., Pressnitz D., Sattler J.H., Kroutil W. Adv. Synth. Catal. 2011;353:3227–3233. [Google Scholar]
- 18.Savile C.K., Janey J.M., Mundorff E.C., Moore J.C., Tam S., Jarvis W.R., Colbeck J.C., Krebber A., Fleitz F.J., Brands J., Devine P.N., Huisman G.W., Hughes G.J. Science. 2010;329:305–309. doi: 10.1126/science.1188934. [DOI] [PubMed] [Google Scholar]
- 19.González-Sabín J., Gotor V., Rebolledo F. Tetrahedron: Asymmetry. 2002;13:1315–1320. [Google Scholar]
- 20.Schrecker A. J. Org. Chem. 1957;22:33–35. [Google Scholar]
- 21.Marco J., Royer J., Husson H.P. Synth. Commun. 1987;17:669–676. [Google Scholar]