The introduction of the novel oral anticoagulants (NOAC), such as the direct Factor Xa-inhibitors rivaroxaban and apixaban and the thrombin-inhibitor dabigatran, have provided an effective and safe alternative to vitamin K antagonists (VKA) for the prevention of thromboembolic complications in patients with non-valvular atrial fibrillation and for prevention and treatment of venous thromboembolism.1 The lack of any need for monitoring and the fixed-dose regimens, because of their predictable pharmacokinetic profile, make these drugs a patient-friendly and practical alternative to VKA. In clinical practice, however, one of the major disadvantages of NOAC is the inability to quantitatively assess the level of anticoagulant effect. This is especially relevant in the case of other medications potentially interfering with the metabolism of these drugs, or when patient adherence is questioned.
We would like to illustrate this issue by presenting the fatal course of a 67-year old patient who was admitted to our hospital. Her medical history consisted of coronary artery bypass grafting and atrial fibrillation. Two months prior to presentation, she received a hip prosthesis after a traumatic femoral fracture. Recovery was complicated by a Staphylococcus aureus wound infection for which she was treated with oral ciprofloxacin and rifampicin for a total duration of three months. Three weeks prior to presentation she was switched from acenocoumarol to rivaroxaban 20 mg once daily because of labile international normalized ratio (INR) levels. At presentation, she complained of a sudden onset of thoracic pain and dyspnea. Physical examination showed a tachypnoeic, sweating patient with hypotension, tachycardia and a body temperature of 35.1 °C; auscultation revealed normal cardiac tones and clear breath sounds. At that time, medication consisted of ciprofloxacin 750 mg bid, rifampicin 150 mg bid and rivaroxaban 20 mg opd. Chest X-ray showed an enlarged cardiac silhouette while electrocardiography was without signs of ischemia. Laboratory tests showed elevated inflammatory markers (C-reactive protein 53 mg/L, leukocytes 19.3 ×109/L with 16.6 neutrophils), mildly elevated troponin-T (89 ng/L) and NT-proBNP (235 pmol/L), and normal renal and hepatic function. An acute coronary syndrome, possibly provoked by an underlying sepsis was suspected, for which acetylsalicylic acid and amoxicillin/clavulanic acid was initiated and admittance to the coronary care unit was arranged. A diagnosis of pulmonary embolism was considered unlikely, because of the current use of rivaroxaban with a prolonged prothrombin time (PT) of 21.6 s (upper limit of normal: 12.5 s) at presentation. Twelve hours later, her condition rapidly deteriorated and died. Upon autopsy, she was found to have extensive central pulmonary embolisms.
A plasma rivaroxaban concentration was measured retrospectively from a stored blood sample taken at presentation. At this time-point, 2 h after oral intake of rivoraxaban 20 mg, the concentration was 178 ng/mL (Figure 1). Although available data on the most effective plasma concentration of rivaroxaban are limited, this peak plasma concentration is just below the 5th percentile of what would be predicted, based on pharmacokinetic and pharmacodynamic studies.2 We, therefore, conclude that this case of fatal pulmonary embolism may be related to sub-therapeutic levels of rivaroxaban, through the interaction of rifampicin with rivaroxaban.
Figure 1.
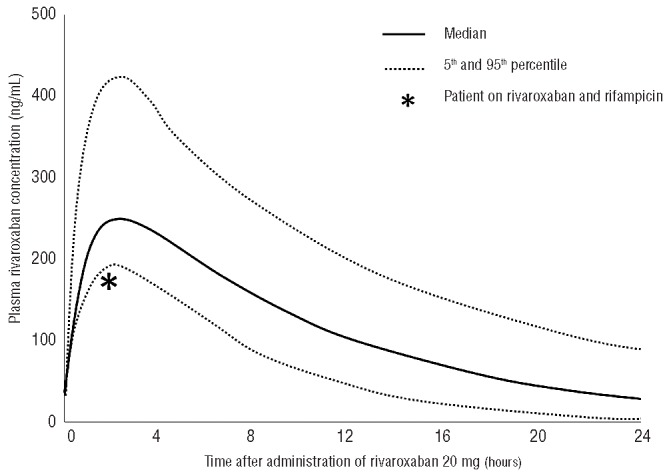
Median (5th and 95th percentiles) plasma levels of rivaroxaban after oral intake of 20 mg, in patients with normal body mass index and normal renal function. Asterix indicates the plasma rivaroxaban concentration in our patient, which was measured from a blood sample taken 2 h after oral intake of 20 mg rivaroxaban. Data on median levels adapted from Mueck et al.2
This case illustrates several critical issues regarding the management of anticoagulant therapy with NOAC, especially when patients are taking additional medication. Firstly, rivaroxaban is, like VKA, a substrate for P-glycoproteïn and CYP3A4. Concomitant use of strong CYP3A4-inducers, such as rifampicin, phenytoin, carbamazepin or phenobarbital can significantly lower rivaroxaban plasma concentrations. This may lead to as much as a 50% reduction in the area under the curve, which is thought to cause a parallel decrease in pharmacodynamic effect.3 The European Medicines Agency (EMA) advocates caution in the concomitant use of rivaroxaban with other CYP3A4-inductors. In these circumstances, it may be prudent to use either low molecular weight heparins or vitamin K antagonists with careful INR monitoring, instead of rivaroxaban.
Secondly, the lack of quantitative assessment of the level of anticoagulation while on rivaroxaban precludes any estimation of the effectiveness of this therapy. A prolonged PT can be used as an insensitive surrogate marker of the use of a Factor Xa-inhibitor; a normal PT almost definitely excludes the presence of a therapeutic plasma concentration of rivaroxaban, although this seems less evident for apixaban. However, there is no linear relationship between a prolonged PT and the intensity of the anticoagulant effect of NOAC. In addition, it is essential to relate the PT to the interval between drawing blood and administration of the medication (Figure 1).2 Measurement of plasma rivaroxaban concentrations or using rivoraxaban-specific anti-Xa tests is promising, but such laboratory assays are still hardly used in daily clinical practice. Importantly, the target therapeutic ranges for the different NOAC have still not been established, nor validated with respect to clinically relevant outcomes.
It is pivotal that all clinicians who are treating patients with NOAC become aware of relevant drug-drug interactions, as well as of the current limited possibilities to assess the level of anticoagulation. Close collaboration with pharmacists appears crucial in this regard. At our hospital, we have recently instituted a dedicated nurse practitioner who is closely involved in the further implementation of the use of NOAC in our patient population. Only through increased awareness of all physicians treating patients with NOAC can an effective treatment with these promising new drugs be achieved, without the risk of potential complications.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Patel MR, Mahaffey KW, Garg J. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91 [DOI] [PubMed] [Google Scholar]
- 2.Mueck W, Lensing AW, Agnelli G, Decousus H, Prandoni P, Misselwitz F. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet. 2011;50(10):675–85 [DOI] [PubMed] [Google Scholar]
- 3.European Medicines Agency Drug product information [cited 2013 June 20th]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000944/WC500057108.pdf


