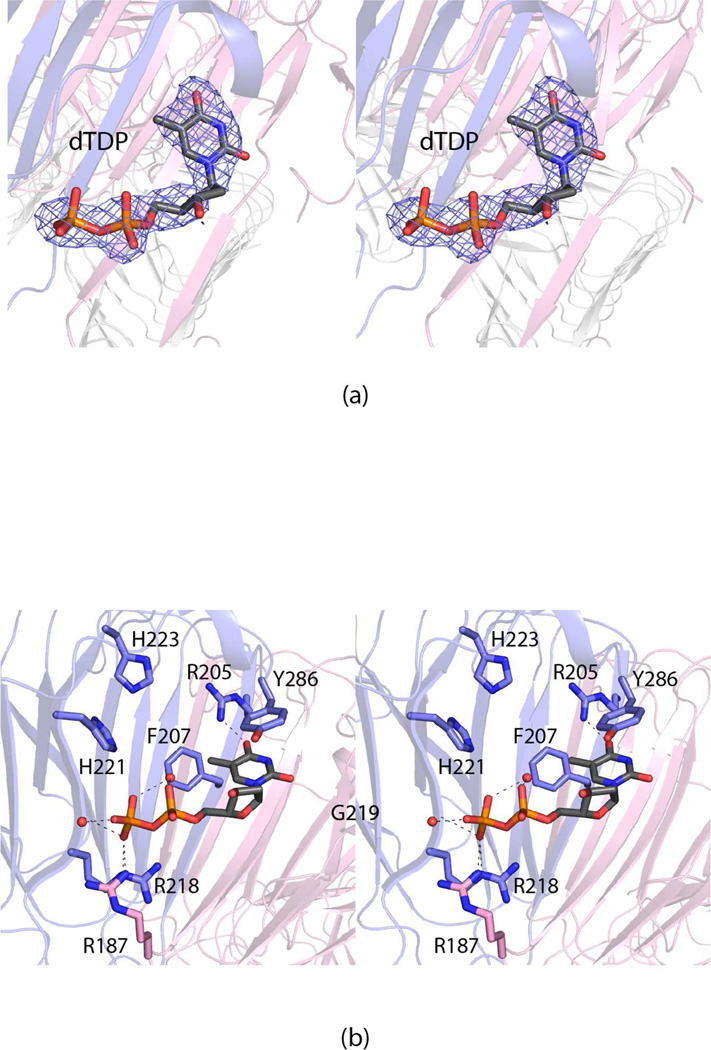
Figure 2.
The active site of the FdtD cupin domain. Electron density corresponding to the bound dTDP is presented in (a). The omit map, contoured at 2σ, was calculated at 2.2 Å resolution with coefficients of the form Fo-Fc, where Fo was the native structure factor amplitude and Fc was the calculated structure factor amplitude (the ligand was not included in the X-ray coordinate file so it did not contribute to the phasing). A close-up view of the region surrounding the dTDP ligand is shown in (b). The dashed lines indicate potential hydrogen bonds. Ordered water molecules are represented by small red spheres. Arg 187, highlighted in pink bonds, is contributed by the second subunit in the dimer as a result of domain swapping.