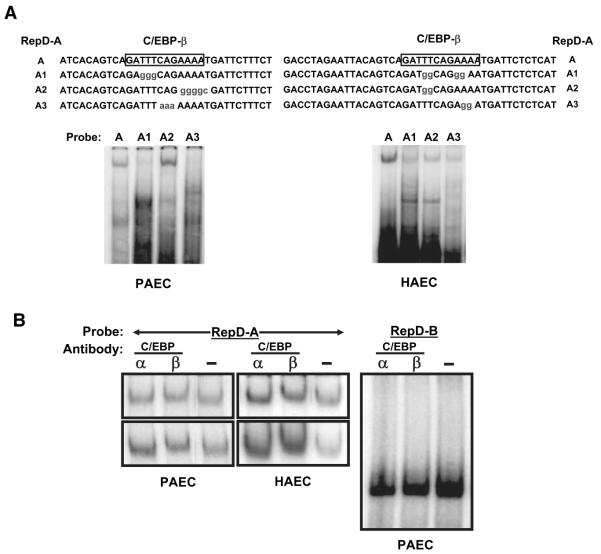
Figure 6.
The IL-1α gene repressor RepD binds to a C/EBP. Nuclear proteins were extracted from porcine (PAEC) or human (HAEC) aortic endothelial cells and incubated with 32P-labeled oligonucleotide probes derived from the RepD-A sequence, as shown in A. The protein–DNA complexes were resolved by nondenaturing polyacrylamide gel electrophoresis and detected by autoradiography of dried gels. A, Nuclear proteins from porcine or human aortic endothelial cells form complexes with RepD-A oligonucleotides but not with RepD-A oligonucleotides containing mutations in the CEBP-β–binding sequence. B, Antibodies to C/EBP-β (lanes marked β) react with protein–RepD-A oligonucleotide complexes and retard migration of the migration through gels. Antibodies against other proteins including C/EBP-α (lanes marked α) or NFATc (data not shown) were without effect. Shown at left side are several examples of experiments using porcine or human porcine aortic endothelial cells probed with the RepD-A probe and anti C/EBP or control (-) antibodies. Antibodies against C/EBP-α or C/EBP-β do not react with the faster-moving complex that forms with RepD-B (right).