Summary
Because of its noninvasive nature and provision of quantitative measures of a wide variety of physiologic parameters, functional magnetic resonance imaging (MRI) shows great potential for research and clinical applications. Over the past decade, application of functional MRI extended beyond detection of cerebral activity, and techniques for abdominal functional MRI evolved. Assessment of renal perfusion, glomerular filtration, interstitial diffusion, and parenchymal oxygenation turned this modality into an essential research and potentially diagnostic tool. Variations in many renal physiologic markers can be detected using functional MRI before morphologic changes become evident in anatomic magnetic resonance images. Moreover, the framework of functional MRI opened a window of opportunity to develop novel pathophysiologic markers. This article reviews applications of some well validated functional MRI techniques, including perfusion, diffusion-weighted imaging, and blood oxygen level–dependent MRI, as well as some emerging new techniques such as magnetic resonance elastography, which might evolve into clinically useful tools.
Introduction
Magnetic resonance imaging (MRI) is a powerful modality. Although functional MRI initially referred mainly to the procedure used to measure brain activity by detecting associated changes in blood flow distribution, today it encompasses a large variety of techniques measuring diverse physiologic markers in many organs. In research, higher magnetic field strengths and sophisticated pulse sequences have opened a window of opportunity to explore new physiologic processes that are detectable by MRI (Table 1). Clinically, MRI has evolved into a sensitive and accurate diagnostic tool that provides information that cannot be achieved noninvasively using other means. Computed tomography (CT) with iodinated contrast agents and renal nuclear imaging using radiolabeled isotopes can also provide renal functional assessment. Whereas CT possesses high spatial and temporal resolution, the spatial resolution of renal nuclear imaging is low and its measurements are semiquantitative. Both techniques require exogenous contrast media and exposure to ionizing radiation. On the contrary, MRI uniquely acquires detailed information without imposing ionizing radiation, and many applications do not necessitate using contrast agents. Although renal functional MRI tools are still largely experimental, understanding their inherent power may facilitate adaptation for clinical practice. Here we review some of the most useful and potentially clinically applicable renal functional MRI methods as well as their prospects for assessing renal pathology.
Table 1.
Key terms in magnetic resonance imaging
| Term | Acronym | Definition |
| Relaxation times | ||
| Longitudinal relaxation time | T1 | A tissue-specific measure of the time that tissue (longitudinal) magnetization takes to restore to its equilibrium value |
| Transverse relaxation time | T2 | A tissue-specific time constant and source of contrast in magnetic resonance images which reflects the time taken for tissue (transverse) magnetization to decay by loss of coherence |
| T2* | A source of magnetic resonance contrast and a measure of (transverse) magnetization decay time, including both tissue-specific mechanisms and field inhomogeneity that contribute to signal decay | |
| Weighted imaging | ||
| T1-weighted imaging | A group of imaging sequences that rely on exogenous contrast or intrinsic T1-relaxtion time properties of different tissues. Images are acquired before the (longitudinal) magnetization of tissues restores to equilibrium. Therefore, tissues with shorter T1 (and faster magnetization recovery) appear bright, whereas tissues with longer T1 (and slower relaxation) remain dark | |
| T2* (and T2)–weighted imaging | Uses the differences of the T2* (or T2) relaxation times as the source of contrast. Regions with shorter T2* (or T2) exhibit a faster decay of MR signal and appear darker, whereas regions with longer relaxation time undergo a slower signal decay and therefore appear brighter. Similar to T1-weighted imaging, the contrast can be enhanced using exogenous contrast media | |
| Diffusion-weighted imaging | DWI | Reflects the level of restriction for water molecule free translocation in biologic tissue. DWI maps provide information about the microstructure of the tissue |
| Perfusion-weighted imaging | PWI | In the kidney, PWI is sensitive to fluid translocation in the microvasculature and tubules, which can be enhanced using endogenous or exogenous contrast media. A quantitative map of perfusion may be generated from a set of perfusion-weighted images |
| Other technical terms | ||
| Dynamic contrast-enhanced imaging: | DCE | Imaging performed during the passage of exogenous contrast agents through a target tissue |
| Arterial spin labeling | ASL | An imaging technique to measure perfusion by acquiring a set of perfusion-weighted images using magnetically labeled inflowing blood as the contrast agent. Labeling takes place by applying a radiofrequency pulse that temporarily alters blood flow magnetization |
| Blood oxygen level–dependent imaging | BOLD | An imaging technique that provides information about blood oxygenation by acquiring a set of T2*-weighted images. BOLD is sensitive to the concentration of deoxyhemoglobin, which acts as an endogenous T2* contrast agent. The higher the concentration of deoxyhemoglobin, the shorter the blood T2* |
| b-value | A parameter that determines diffusion sensitivity in DWI. The higher the b-value, the stronger the diffusion weighting | |
| Apparent diffusion constant | ADC | A quantitative measure of water diffusivity in biologic tissue. Low ADC values may reflect diffusion restriction by membranes or other microstructures |
| Fractional anisotropy | FA | A measure of directional diffusivity within a range of 0–1. FA=0 demonstrates isotropic diffusion (in all direction), whereas FA=1 reflects diffusion along a single direction but restricted in all other directions (e.g., tubules) |
Renal Perfusion
Renal hemodynamic parameters are important markers of many renal pathologic conditions. Although their assessment has been more common in renovascular disease, such as renal artery stenosis, it can be useful in other pathologic conditions that affect the microvasculature, renal blood flow, vascular resistance, or permeability such as CKD, hypertension, metabolic syndrome, diabetes, and sepsis (1). Moreover, perfusion measurement may help guide kidney transplant management and treatment of renal lesions (Table 2).
Table 2.
Potential applications for renal functional MRI
| Imaging Type | Acronym | Imaging Marker(s) | Biologic Marker | Potential Clinical Applications (Reference) | Limitations |
| Dynamic contrast–enhanced imaginga | DCE | Perfusion | Hemodynamic | Renal artery stenosis (4) | Nephrotoxicity of contrast agent |
| Blood volume | CKD | Lack of standard imaging and analysis protocols | |||
| Mean transit time | Ischemia-reperfusion | ||||
| Hypertension | |||||
| Arterial spin labeling | ASL | Perfusion | Hemodynamic | Renal tumors (6) | Complex imaging sequence |
| Allograft assessment (7) | Short t1/2 of the labeled blood | ||||
| Pre/diabetes (8) | |||||
| Diffusion-weighted imaging and diffusion tensor imaging | DWI, DTI | Apparent diffusion constant | Morphologic and functional (flow, fluid exchange and reabsorption) | Renal cell carcinoma | Complex interpretation |
| Pseudo/diffusivity | Renal artery stenosis (18) | lack of standard imaging and analysis protocols | |||
| Fluid fraction | Allograft assessment (7,22) | ||||
| Fractional anisotropy | Diabetic nephropathy | ||||
| Ureteral obstruction | |||||
| CKD | |||||
| Blood oxygen level–dependent imaging | BOLD | Relaxivity (oxygenation level) | Oxygenation | Renal artery stenosis (34) | Complex interpretation |
| Response to challenge | Oxygen-dependent tubular transport function | Diabetes (30) | Lack of standard imaging and analysis protocols | ||
| Allograft assessment (27) | |||||
| Acute and CKD | |||||
| Magnetic resonance elastography | MRE | Stiffness | Tissue elasticity | Allograft assessment | Sensitive to hemodynamics |
| Fibrosis | Renal artery stenosis | Requires hardware and analytical software | |||
| Turgor | Ureteral obstruction | ||||
| Spectroscopic molecular imaging | 31P | ATP generation | AKI | Time-consuming | |
| Renal allograft (46) | Technically complicated | ||||
| Fat fraction | Fat-to-water ratio | Visceral lipid | Obesity | Needs further validation in the kidney | |
| Metabolic syndrome | |||||
| Diabetes |
Depending on the model and the type of contrast agent, measurement of other markers (such as vascular permeability and filtration fraction) could be possible.
Hemodynamic parameters are often derived from mathematical models, which link them to dynamic changes of MR signal intensity during transition of the contrast-enhancement agent through the tissue. Lack of a standard protocol for perfusion measurement, as well as complicated fluid dynamics in the kidney that require more elaborate analytical models than in many other organs, have restricted wide clinical application of this technique, yet it has been used in a large number of experimental basic and clinical studies. The most common magnetic resonance (MR) approaches for perfusion measurement include dynamic contrast-enhanced (DCE) and arterial spin labeling (ASL).
DCE-MRI
Hemodynamic measurements with DCE-MRI rely on exogenous tracers that affect the blood MR characteristics. A tracer bolus alters two independent MR time constants and consequently creates dynamic contrast that can be detected using T1- and T2*-weighted perfusion measurements. Tissue carrying a contrast agent that shortens the T1 and T2* relaxation times appears brighter in T1-weighted and darker in T2*-weighted images. MR signal intensity can be translated to contrast media concentration in order to generate a concentration-time curve for any region of interest (Figure 1). A concentration-time curve depicts vascular, proximal, and distal tubular phases in the cortex, whereas only vascular and tubular (Loop of Henle) phases are usually distinguishable in the medulla within the same timeframe. These curves carry important functional information about subcompartments of the kidney and are the primary sources of data for perfusion models to calculate hemodynamic parameters. Generally, a DCE-MRI acquisition continues for 3–10 minutes after administration of the contrast bolus. Abdominal imaging artifacts, including motion artifacts perpetuated by respiration, add to the complexity of kidney perfusion measurements. Although several methods may address respiratory motion artifacts, the most commonly practiced method utilizes breath-holding, particularly during the early rapid passage of the contrast agent in blood vessels (vascular phase). In the abdomen, T1-weighted imaging is the method of choice because unlike T2*-weighted perfusion measurement, it is insensitive to tracer extravasation and therefore affords information about vascular permeability.
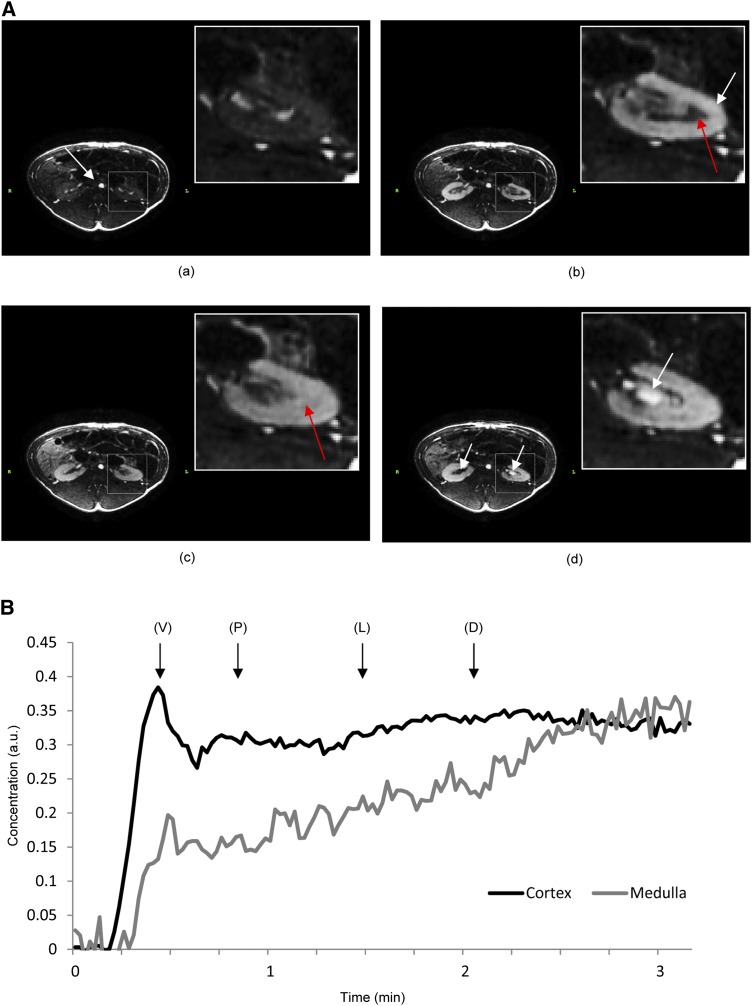
Figure 1.
Dynamic contrast-enhanced magnetic resonance imaging–derived images in a swine unilateral renal artery stenosis. (A) The stenotic (right) and the contralateral (left) kidneys at baseline (a) and at the vascular (b) and tubular (c) phases. High cortical blood flow results in high contrast between the cortex and medulla during the vascular phase. (a and b) The white arrow shows the arrival of the contrast bolus in the aorta (a) and in the cortex (b) (during the contrast agent transition), whereas the red arrow shows the medulla. (c) The physiologically hypoperfused medulla shows visible enhancement mainly during the tubular phase. (d) Finally, as contrast media wash out from the tissue, their appearance in the stenotic kidney calyx is delayed compared with the contralateral kidney. (B) Typical magnetic resonance concentration-time curves, with vascular, proximal tubular, Loop of Henle, and distal tubular transitions. D, distal tubular; L, Loop of Henle; P, proximal tubular; V, vascular.
Ultrasmall paramagnetic iron oxide (USPIO) particles and gadolinium-chelates have been used as contrast agents for renal hemodynamic assessments (2,3). USPIO particles, tens of nanometers in diameter, remain initially intravascular and are eliminated from the blood pool by the reticuloendothelial system. Therefore, models originally developed for brain perfusion measurement, which assume no contrast agent leakage from the microvasculature, can be applied for description of USPIO kinetics. Such models with a single vascular compartment are simple in nature, but do not afford information about kidney filtration. There is currently no Food and Drug Administration (FDA)–approved USPIO available and its use remains experimental.
On the contrary, gadolinium-chelate may diffuse out of the microvasculature, and a description of its kinetics demands more sophisticated models with two or more compartments (e.g., vascular, tubular, and extravascular), often describing the exchange rates between compartments (e.g., GFR or vascular permeability). Renal perfusion (ml/100 ml tissue per minute), blood volume (a fraction of total parenchymal volume), plasma mean transit time (seconds), time-to-peak (seconds), and regional filtration fraction (ml/100 ml tissue per minute) are useful functional parameters derived from multicompartmental models. Selection of a suitable mathematical model is based on the desired choice of markers and the validity of its underlying assumptions for a specific pathologic condition. Importantly, data analysis mandates selection of the region of interest in accordance with the underlying assumptions. For example, models that presume conservation of the contrast media require probing the entire kidney (in which the agent usually remains throughout the scan) and provide global information, whereas those that allow for contrast translocation during scanning permit more localized information.
The challenge for MR perfusion is the identification of significant hemodynamic and functional impairments. Speculatively, DCE may be useful in pathologic conditions that affect parenchymal perfusion through dysregulation, atherosclerosis, microvascular rarefaction, or even inflammation-associated renal function impairments. Yet DCE is reliable mostly in detecting pronounced hemodynamic alterations such as renal blood flow impairment at renal artery stenosis ≥80% (4), above which a severe stenosis compromises flow (5). Such drastic changes may also occur in treatment of intrinsically hyperperfused tumors or during ischemia-reperfusion.
ASL
ASL-MRI is noninvasive and utilizes arterial blood as the contrast tracer. This method is particularly attractive as an alternative to DCE for renal applications because it eliminates the need for exogenous contrast media with possible adverse effects, particularly in patients with compromised kidney function. However, due to some limitations, ASL is primarily used for cortical perfusion measurement, and technical complexity has restricted its application primarily to research.
Using ASL, perfusion is measured by acquiring a set of labeled (tagged) and nonlabeled (control) images. Spin labeling (or tagging) involves inversion of the magnetization of arterial blood using a radiofrequency pulse. Once the labeled blood reaches the kidney and replaces the untagged blood, it reduces the intensity of the MR signal. Subtracting labeled from control images provides perfusion-weighted images with a low signal to noise ratio (SNR). Absolute perfusion can be quantified from a set of perfusion-weighted images with various delays between tagging and acquisition. The total ASL acquisition time depends on the number of perfusion-weighted images acquired, from tens of seconds to several minutes.
Thus far, ASL perfusion measurements in renal cell carcinoma (RCC) and allografts have shown promise. In patients with advanced RCC, ASL can evaluate outcomes of therapy, taking advantage of the high perfusion in tumors (6). Moreover, perfusion might indicate the stability of renal allograft function. Transplanted kidneys often have lower perfusion than native kidneys, yet a severe decline could indicate ischemic injury and functional deterioration (7). A recent study showed that ASL is also promising for assessing renal perfusion in patients with metabolic syndrome and detecting hemodynamic responses to pharmacologic interventions (8).
GFR
The fundamental assumption in GFR estimation using contrast-enhanced imaging is that the agent has a filtration rate similar to the body fluids. Methodologies applied to estimate GFR from DCE-MR images all rely on similar concepts to differentiate the extracted (filtered) tracer from that in the blood. Contrast media in the blood rapidly circulate through the parenchymal vasculature, but slowly accumulate and flow in the tubules when extracted. Whereas earlier studies utilized mathematically simple models, such as graph-based Patlak (9) and Upslope (10) methods, sophisticated models involving three or more compartments have evolved in recent years (11). The usefulness of noninvasive single-kidney GFR measurements prompted consideration of models with additional compartments, which afford a broader range of hemodynamic markers. A separable multicompartment model may be more reliable than the widely used Patlak method for estimation of single-kidney GFR (12), yet the correlation between standard radioisotope measures and the Patlak method was stronger compared with the compartmental approach (13). Nevertheless, there is yet no reference standard for a multicompartmental model.
A concern for GFR measurement, particularly in patients with CKD, is the potential adverse effects of gadolinium-based MR contrast agents like nephrogenic systemic fibrosis (14), which is linked to a high cumulative dose of gadolinium (15). Notably, although clinical protocols utilize 5–20 mM of gadolinium-based contrast agents, GFR measurements with a dose as low as 1.4–2.8 mM are feasible (16), yet a dose of gadolinium that is completely safe to use in patients with severely impaired renal function has not been identified.
Diffusion-Weighted Imaging
Diffusion-weighted imaging (DWI) MRI is a powerful method that provides several parameters that describe the restriction imposed by microstructures on Brownian random motion of water molecules in biologic tissues (Figure 2), and is thereby sensitive to morphologic changes. The method requires no exogenous contrast agent and is therefore clinically applicable; the acquisition times (<1 minute) are usually short enough to collect several slices within few breath-holds. The apparent diffusion constant (ADC), the DWI quantitative index, describes the average diffusivity of water molecules in all three directions, and is determined by fitting the curve of DWI-MR signal intensity versus b-values, a parameter that determines the diffusion weighting, to an exponentially decaying curve. Despite its high potential, the application of DWI has been limited to research. Lack of a standard protocol, ADC dependency on b-values, and complexity of result interpretation are some of the issues restricting the renal application of this technique.
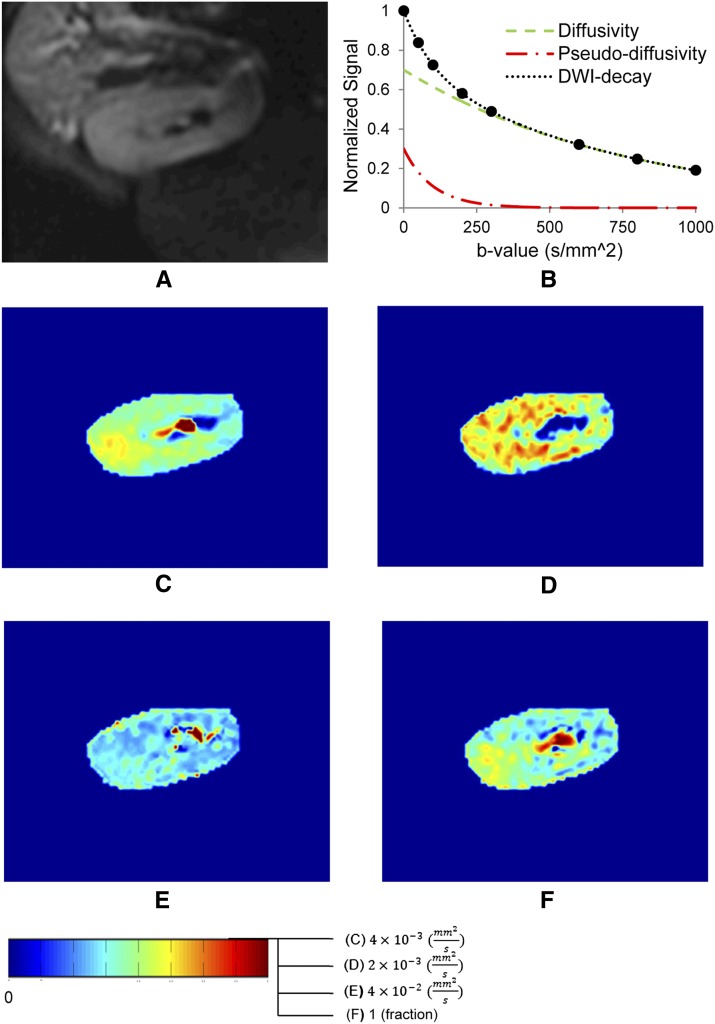
Figure 2.
Diffusion-weighted magnetic resonance imaging, with different maps that reflect the diverse parameters that can be derived. (A) Anatomic MR image. (B) Biexponential signal decay consistent with pseudodiffusivity (fast decaying perfusion and tubular flow dependence) and diffusivity (slowly decaying pure tissue diffusion dependence) components. (C) Apparent diffusion constant map from monoexponential decay model. (D) Pure tissue diffusivity map. (E) Pseudodiffusivity map representing tubular fluid and microvascular blood velocity. (F) Perfusion fraction (fluid fraction) map calculated using a intravoxel incoherent motion biexponential decay model. DWI, diffusion-weighted imaging.
DWI has been used to evaluate a variety of renal pathologies, including lesions, acute and chronic disease, and allografts. Most pathologic conditions, including acute and chronic failure, chronic ureteral obstruction, and pyelonephritis, reduce ADC in the cortex and medulla compared with healthy kidneys (17). DWI differentiated stable from deteriorating allograft function in transplant patients, whereas ADC correlates with GFR in patients with renal artery stenosis (18). Decreases in ADC have been reported in RCC, although inflammatory lesions impose similar diffusion restrictions (19). A lower ADC might be consequent to increased cellularity that imposes barriers to free diffusion of water (19) or to accumulation of fibroblasts (20).
The simplicity of the analytical model and feasibility of determination of ADC using two b-values allowed this technique to initially rely on a monoexponential decay model. However, recent studies have emphasized the capabilities of a biexponential decay approach implementing an intravoxel incoherent motion model, which discerns the contributions of diffusion and flow to the decay of DWI signal by their different b-value dependency (21). For b-values, >300 s/mm2 diffusion is the dominant mechanism of decay of the MR signal, whereas it is attributed to tubular flow, perfusion, and diffusion for smaller b-values. The model has decreased the variations in diffusion values reported in the kidney, but at the same time has added to the complexity of result interpretation. Intravoxel incoherent motion parameters are sensitive to tissue structure, fractional (tubular and vascular) fluid content, and their velocities. These markers have been used in early experimental studies to distinguish benign renal lesions from malignant tumors, because increased vascularity in malignant tumors is believed to increase perfusion fraction and decrease tissue diffusivity (7). Yet, given the complexity of kidney and interrelated fluid dynamics, further validation studies are necessary.
Diffusion tensor imaging (DTI) provides greater details than DWI about diffusion of free water in tissues. This information can be translated into elaborate markers such as fractional anisotropy (FA), which indicates whether water molecules are free to diffuse equally in all directions (isotropic diffusion FA=0), or are restricted in some (anisotropic diffusion). FA=1 corresponds to diffusion only along one orientation. Graphical maps to illustrate tissue microstructure were originally developed to detect integrity of neural tracts, and are therefore termed tractography. Several recent studies have reported higher sensitivity of FA compared with ADC to detect renal pathomorphology (22,23). Moreover, early application of DTI tractography revealed impaired medullary microstructure in dysfunctional allograft kidneys, in contrast to its highly organized microstructure in healthy kidneys (22). The utility and potential applications of this novel technique remain to be explored. Some drawbacks to DTI are time-consuming acquisitions and data processing, which may take hours.
Blood Oxygen Level–Dependent MRI
Blood oxygen level–dependent (BOLD) MRI is a unique tool that is currently clinically available for renal imaging research. The technique was initially developed for neuroimaging but found applications in different tissues and pathologic conditions due to its noninvasive nature (24). BOLD is sensitive to the blood concentration of paramagnetic deoxyhemoglobin, which acts as a MR contrast agent. Increased concentration of deoxyhemoglobin results in shorter T2* (faster MR signal decay). The BOLD index, R2* (1/T2*), is considered a measure of tissue oxygenation level or hypoxia, based on the assumption that blood and tissue oxygenation are at tight equilibrium. Notably, some experimental investigations have challenged this assumption (25). Moreover, some pathologic conditions, like fibrosis, may restrict oxygen diffusion and prevent equilibrium (Figure 3), resulting in an oxygen gradient across the microvascular lumen, in which case blood oxygenation no longer represents tissue oxygenation. Nevertheless, BOLD remains the most popular technique to experimentally measure tissue oxygenation in vivo. The acquisition time is relatively short (1–5 minutes) and collected over several breath-holds. Its sensitivity to detect differences in oxygenation improves at higher fields, because R2* magnitude is scaled by magnetic field strength (26).
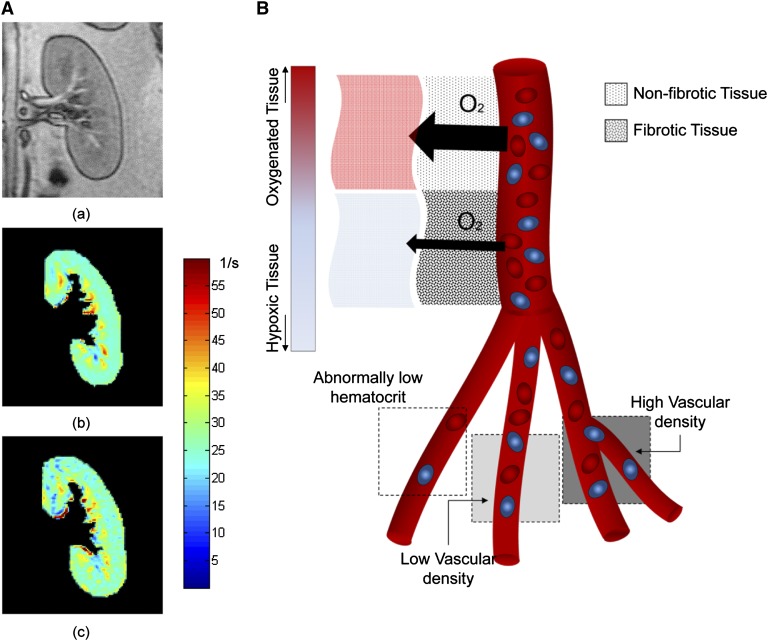
Figure 3.
Renal BOLD MRI and physiological restricting factors. (A) Representative anatomic reference (a) and BOLD maps before (b) and after (c) administration of furosemide. Furosemide reduces oxygen-dependent tubular transport in the medulla and improves medullary oxygenation (drops on the scale toward green-blue shades). The response to furosemide is measurable by comparing the areas of the hypoxic regions and/or the change in average R2* magnitude. (B) Parameters that may affect BOLD magnetic resonance images. Fibrotic tissues can restrict oxygen exchange between the microvasculature and tissue, so that due to low oxygen diffusivity the vascular oxygenation (sampled by BOLD) remains high despite tissue hypoxia. The three bottom squares represent relative contrast in a T2*-weighted image, which can be affected by density of capillaries or hemoglobin (e.g., hematocrit). Higher concentration of hemoglobin results in faster decay of the magnetic resonance signal and gives rise to dark regions interpreted as hypoxic tissue, without necessarily representing tissue oxygenation. BOLD, blood oxygen level–dependent.
Renal oxygenation has been used as an index of kidney allograft dysfunction and acute transplant rejection. Lower medullary R2* values in acute renal transplant rejection (27) may represent higher oxygen bioavailability in the intrinsically hypoxic medulla secondary to impaired metabolism and halted tubular transport function (28). BOLD has also been used to study renal oxygenation in several animal models of diabetic nephropathy (29,30). Renal oxygenation likely changes in only advanced disease, because lower oxygenation is observed in the outer medulla of rats with diabetic nephropathy (29), but not in prediabetic, obese swine (31).
In a model of acute renal arterial occlusion, BOLD revealed that increases in R2* paralleled the level of occlusion and reduction of renal blood flow (32). Indeed, patient with significant (33), but not moderate (34), renal artery stenosis show decreased renal oxygenation compared with essential hypertension. In addition to renal hypoxia, the use of pharmaceutical maneuvers permits assessment of oxygen-dependent tubular transport function (35). Furosemide, an inhibitor of the Na/K/Cl cotransporter in the thick ascending limb, has been used to evaluate medullary tubular function in a variety of renal pathologic conditions (36). Selective challenges for cortical transport activity need to be developed in order to probe cortical tubular function.
A recent study showed that the basal cortical and medullary BOLD signal was nonspecific for discriminating patients in a large cohort with diverse chronic renal diseases (37). These observations subsequently raised methodological questions (38), because BOLD uses deoxyhemoglobin as the contrast agent, and pathologic conditions (e.g., anemia or ischemia) that affect the hematocrit, microvascular density, or regional renal blood volume may nonuniformly influence the signal in a heterogynous population (39). Moreover, BOLD measurements are prone to susceptibility artifacts caused by bowel gas, which increases R2* values and might be erroneously considered as hypoxic regions. The severity of the artifact increases at higher magnetic fields, and warrants careful selection of regions of interest. Notably, analytical methods utilizing histogram-based tools that recognize the variability of the BOLD signal intensity within the regions of interest may be capable of detecting differences (40) and introduce new markers beyond the traditional mean of R2* value, such as their distribution (41,42).
Emerging Methods
Elastography
Magnetic resonance elastography (MRE) is a noninvasive imaging technique that utilizes translocation of mechanical shear waves to estimate tissue stiffness (Figure 4). Based on the assumption that excessive extracellular matrix deposition in fibrotic tissues increases their stiffness, MRE-derived stiffness has been used as an index of fibrosis. The feasibility of using MRE in the kidney has been demonstrated in transplant patients (43) and in swine renal artery stenosis (44,45). Interestingly, studies utilizing graded renal ischemia demonstrated that hemodynamic modulation of renal cortical stiffness hampers discrimination of fibrotic from nonfibrotic kidneys (44). However, MRE is capable of detecting fibrosis in the intrinsically hypoperfused medulla (45), which is less dependent on perfusion pressure. Understanding of underlying physiologic processes and development of appropriate models will likely increase the use of this novel technique (e.g., for monitoring evolution or regression of kidney fibrosis in abnormalities such as ureteral obstruction).
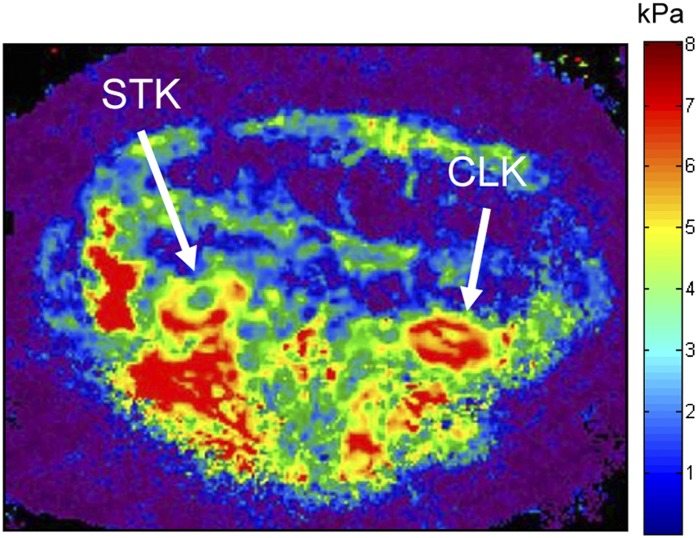
Figure 4.
Magnetic resonance elastography in swine unilateral renal artery stenosis. Lower stiffness (less red color) in the cortex of the stenotic kidney (right) compared with the contralateral kidney (left), despite greater fibrosis, is a consequence of lower perfusion pressure (and turgor) distal to the stenosis. CLK, contralateral kidney; STK, stenotic kidney.
Molecular Imaging
31P MR spectroscopy was one of the earliest applications of molecular MR in kidney. Renal failure is often accompanied by a loss of ATP and progressive formation of inorganic phosphorus. Therefore, the ratio of phosphomonoesters to inorganic phosphorus is used as a marker for renal metabolism and allograft viability (46). Recent advances have improved the quality of data acquisition in the kidney by utilizing chemical shift imaging; however, due to low SNR, the imaging resolution remains far lower than conventional MRI (47).
Fat Fraction
Fat fraction imaging-based methods used to quantify renal fat content (48) rely on the spectroscopic imaging method originally proposed by Dixon in 1984 (49). The technique involves acquiring in-phase (water + fat) and out-of-phase (water − fat) images to generate water-only and fat-only images (Figure 5). In the liver, quantification of hepatic fat becomes inaccurate in the presence of high fat content (50). Due to its lower fat fraction, it is unlikely that this issue limits the application of this method in the kidney. Nevertheless, fat quantification using MR is new in kidney applications and further investigations are needed to evaluate this method, which may in turn allow evaluation of the significance and clinical or pathologic correlates of renal adiposity.
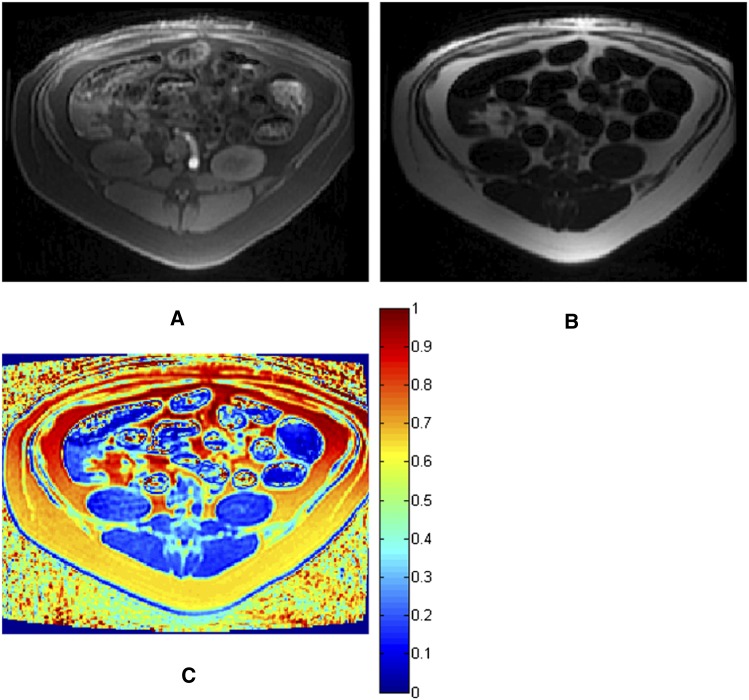
Figure 5.
Fat quantification by magnetic resonance imaging. Water-only (A) and fat-only (B) images and the fat-ratio map (C) calculated from in-phase and out-of-phase images (not shown).
Functional MRI in the kidney has come a long way and has become an indispensable research tool. All of the above-mentioned methods are noninvasive and can potentially be translated to clinical protocols. However, the lack of standardized acquisition and analysis protocols often limits functional MRI techniques. Some tools, such as DCE perfusion and GFR measurements, have already been extensively investigated in clinical trials. Nevertheless, more sophisticated and elaborate models are needed to address current limitations. BOLD and ASL are particularly promising techniques with potentially broad future clinical applications. Like all imaging techniques, they are based on assumptions, which might not always faithfully reflect the conditions in vivo and may introduce errors or impose limitations to the applicability of the techniques. DWI, DTI, and the emerging methods are powerful techniques that can provide a diverse range of biologic markers. However, additional studies are required to fully understand these markers and their association with pathologic conditions. Despite some shortcomings and limitations, functional MRI remains one of the most powerful and versatile imaging approaches available today. In particular, these functional methods provide valuable information and will remain essential for future research and potential clinical applications.
Disclosures
S.C.T. and L.O.L. have received institutional support from Stealth Peptides Inc. L.O.L. serves on the advisory board of Stealth Peptides Inc.
Acknowledgments
This research was partly supported by the National Institutes of Health (Grants DK073608, HL77131, and HL085307) and the Mayo Clinic Center for Regenerative Medicine.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Prowle JR, Molan MP, Hornsey E, Bellomo R: Measurement of renal blood flow by phase-contrast magnetic resonance imaging during septic acute kidney injury: A pilot investigation. Crit Care Med 40: 1768–1776, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Aumann S, Schoenberg SO, Just A, Briley-Saebo K, Bjørnerud A, Bock M, Brix G: Quantification of renal perfusion using an intravascular contrast agent (part 1): Results in a canine model. Magn Reson Med 49: 276–287, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Dujardin M, Sourbron S, Luypaert R, Verbeelen D, Stadnik T: Quantification of renal perfusion and function on a voxel-by-voxel basis: A feasibility study. Magn Reson Med 54: 841–849, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Schoenberg SO, Aumann S, Just A, Bock M, Knopp MV, Johansson LO, Ahlstrom H: Quantification of renal perfusion abnormalities using an intravascular contrast agent (part 2): Results in animals and humans with renal artery stenosis. Magn Reson Med 49: 288–298, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Lerman LO, Schwartz RS, Grande JP, Sheedy PF, Romero JC: Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol 10: 1455–1465, 1999 [DOI] [PubMed] [Google Scholar]
- 6.de Bazelaire C, Alsop DC, George D, Pedrosa I, Wang YY, Michaelson MD, Rofsky NM: Magnetic resonance imaging-measured blood flow change after antiangiogenic therapy with PTK787/ZK 222584 correlates with clinical outcome in metastatic renal cell carcinoma. Clin Cancer Res 14: 5548–5554, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Heusch P, Wittsack HJ, Heusner T, Buchbender C, Quang MN, Martirosian P, Bilk P, Kröpil P, Blondin D, Antoch G, Lanzman RS: Correlation of biexponential diffusion parameters with arterial spin-labeling perfusion MRI: results in transplanted kidneys. Invest Radiol 48: 140–144, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Ritt M, Janka R, Schneider MP, Martirosian P, Hornegger J, Bautz W, Uder M, Schmieder RE: Measurement of kidney perfusion by magnetic resonance imaging: Comparison of MRI with arterial spin labeling to para-aminohippuric acid plasma clearance in male subjects with metabolic syndrome. Nephrol Dial Transplant 25: 1126–1133, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Hackstein N, Heckrodt J, Rau WS: Measurement of single-kidney glomerular filtration rate using a contrast-enhanced dynamic gradient-echo sequence and the Rutland-Patlak plot technique. J Magn Reson Imaging 18: 714–725, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Montet X, Ivancevic MK, Belenger J, Jorge-Costa M, Pochon S, Pechère A, Terrier F, Vallée JP: Noninvasive measurement of absolute renal perfusion by contrast medium-enhanced magnetic resonance imaging. Invest Radiol 38: 584–592, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Lee VS, Rusinek H, Bokacheva L, Huang AJ, Oesingmann N, Chen Q, Kaur M, Prince K, Song T, Kramer EL, Leonard EF: Renal function measurements from MR renography and a simplified multicompartmental model. Am J Physiol Renal Physiol 292: F1548–F1559, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Sourbron SP, Michaely HJ, Reiser MF, Schoenberg SO: MRI-measurement of perfusion and glomerular filtration in the human kidney with a separable compartment model. Invest Radiol 43: 40–48, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Buckley DL, Shurrab AE, Cheung CM, Jones AP, Mamtora H, Kalra PA: Measurement of single kidney function using dynamic contrast-enhanced MRI: Comparison of two models in human subjects. J Magn Reson Imaging 24: 1117–1123, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Perazella MA: Nephrogenic systemic fibrosis, kidney disease, and gadolinium: Is there a link? Clin J Am Soc Nephrol 2: 200–202, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Kallen AJ, Jhung MA, Cheng S, Hess T, Turabelidze G, Abramova L, Arduino M, Guarner J, Pollack B, Saab G, Patel PR: Gadolinium-containing magnetic resonance imaging contrast and nephrogenic systemic fibrosis: A case-control study. Am J Kidney Dis 51: 966–975, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Rusinek H, Lee VS, Johnson G: Optimal dose of Gd-DTPA in dynamic MR studies. Magn Reson Med 46: 312–316, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Thoeny HC, De Keyzer F: Diffusion-weighted MR imaging of native and transplanted kidneys. Radiology 259: 25–38, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Xu YF, Wang XY, Jiang XX: Relationship between the renal apparent diffusion coefficient and glomerular filtration rate: Preliminary experience. J Magn Reson Imaging 26: 678–681, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Goyal A, Sharma R, Bhalla AS, Gamanagatti S, Seth A: Diffusion-weighted MRI in inflammatory renal lesions: All that glitters is not RCC! Eur Radiol 23: 272–279, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Togao O, Doi S, Kuro-o M, Masaki T, Yorioka N, Takahashi M: Assessment of renal fibrosis with diffusion-weighted MR imaging: Study with murine model of unilateral ureteral obstruction. Radiology 255: 772–780, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M: Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168: 497–505, 1988 [DOI] [PubMed] [Google Scholar]
- 22.Hueper K, Gutberlet M, Rodt T, Gwinner W, Lehner F, Wacker F, Galanski M, Hartung D: Diffusion tensor imaging and tractography for assessment of renal allograft dysfunction-initial results. Eur Radiol 21: 2427–2433, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Gaudiano C, Clementi V, Busato F, Corcioni B, Orrei MG, Ferramosca E, Fabbri E, Berardi P, Santoro A, Golfieri R: Diffusion tensor imaging and tractography of the kidneys: Assessment of chronic parenchymal diseases. Eur Radiol 23: 1678–1685, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Prasad PV, Edelman RR, Epstein FH: Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation 94: 3271–3275, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Evans RG, Leong CL, Anderson WP, O’Connor PM: Don’t be so BOLD: Potential limitations in the use of BOLD MRI for studies of renal oxygenation. Kidney Int 71: 1327–1328, author reply 1328, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Gloviczki ML, Glockner J, Gomez SI, Romero JC, Lerman LO, McKusick M, Textor SC: Comparison of 1.5 and 3 T BOLD MR to study oxygenation of kidney cortex and medulla in human renovascular disease. Invest Radiol 44: 566–571, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadowski EA, Djamali A, Wentland AL, Muehrer R, Becker BN, Grist TM, Fain SB: Blood oxygen level-dependent and perfusion magnetic resonance imaging: Detecting differences in oxygen bioavailability and blood flow in transplanted kidneys. Magn Reson Imaging 28: 56–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thoeny HC, Zumstein D, Simon-Zoula S, Eisenberger U, De Keyzer F, Hofmann L, Vock P, Boesch C, Frey FJ, Vermathen P: Functional evaluation of transplanted kidneys with diffusion-weighted and BOLD MR imaging: Initial experience. Radiology 241: 812–821, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Ries M, Basseau F, Tyndal B, Jones R, Deminière C, Catargi B, Combe C, Moonen CWT, Grenier N: Renal diffusion and BOLD MRI in experimental diabetic nephropathy. Blood oxygen level-dependent. J Magn Reson Imaging 17: 104–113, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Yin WJ, Liu F, Li XM, Yang L, Zhao S, Huang ZX, Huang YQ, Liu RB: Noninvasive evaluation of renal oxygenation in diabetic nephropathy by BOLD-MRI. Eur J Radiol 81: 1426–1431, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Li ZL, Woollard JR, Wang SM, Korsmo MJ, Ebrahimi B, Grande JP, Textor SC, Lerman A, Lerman LO: Increased glomerular filtration rate in early metabolic syndrome is associated with renal adiposity and microvascular proliferation. Am J Physiol Renal Physiol 301: F1078–F1087, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juillard L, Lerman LO, Kruger DG, Haas JA, Rucker BC, Polzin JA, Riederer SJ, Romero JC: Blood oxygen level-dependent measurement of acute intra-renal ischemia. Kidney Int 65: 944–950, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Gloviczki ML, Glockner JF, Crane JA, McKusick MA, Misra S, Grande JP, Lerman LO, Textor SC: Blood oxygen level-dependent magnetic resonance imaging identifies cortical hypoxia in severe renovascular disease. Hypertension 58: 1066–1072, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gloviczki ML, Glockner JF, Lerman LO, McKusick MA, Misra S, Grande JP, Textor SC: Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension 55: 961–966, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warner L, Glockner JF, Woollard J, Textor SC, Romero JC, Lerman LO: Determinations of renal cortical and medullary oxygenation using blood oxygen level-dependent magnetic resonance imaging and selective diuretics. Invest Radiol 46: 41–47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebrahimi B, Li ZL, Eirin A, Zhu XY, Textor SC, Lerman LO: Addition of endothelial progenitor cells to renal revascularization restores medullary tubular oxygen consumption in swine renal artery stenosis. Am J Physiol Renal Physiol 302: F1478–F1485, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michaely HJ, Metzger L, Haneder S, Hansmann J, Schoenberg SO, Attenberger UI: Renal BOLD-MRI does not reflect renal function in chronic kidney disease. Kidney Int 81: 684–689, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Inoue T, Kozawa E, Okada H, Suzuki H: Is there no future for renal BOLD-MRI? [quest] Kidney Int 82: 934–, author reply 935., 2012 [DOI] [PubMed] [Google Scholar]
- 39.Fine LG, Dharmakumar R: Limitations of BOLD-MRI for assessment of hypoxia in chronically diseased human kidneys. Kidney Int 82: 934–935, author reply 935, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Michaely HJM: Renal BOLD-MRI does not reflect renal function in chronic kidney disease; the author replies. Kidney Int 82: 935, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Ebrahimi B, Gloviczki M, Woollard JR, Crane JA, Textor SC, Lerman LO: Compartmental analysis of renal BOLD MRI data: Introduction and validation. Invest Radiol 47: 175–182, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saad A, Crane JA, Glockner JF, Herrmann SM, Friedman H, Ebrahimi B, Lerman LO, Textor SC: Human renovascular disease: Estimating fractional tissue hypoxia to analyze blood oxygen level-dependent MR. Radiology 268: 770–778, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee CU, Glockner JF, Glaser KJ, Yin M, Chen J, Kawashima A, Kim B, Kremers WK, Ehman RL, Gloor JM: MR elastography in renal transplant patients and correlation with renal allograft biopsy: A feasibility study. Acad Radiol 19: 834–841, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warner L, Yin M, Glaser KJ, Woollard JA, Carrascal CA, Korsmo MJ, Crane JA, Ehman RL, Lerman LO: Noninvasive in vivo assessment of renal tissue elasticity during graded renal ischemia using MR elastography. Invest Radiol 46: 509–514, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korsmo MJ, Ebrahimi B, Eirin A, Woollard JR, Krier JD, Crane JA, Warner L, Glaser K, Grimm R, Ehman RL, Lerman LO: Magnetic resonance elastography noninvasively detects in vivo renal medullary fibrosis secondary to swine renal artery stenosis. Invest Radiol 48: 61–68, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seto K, Ikehira H, Obata T, Sakamoto K, Yamada K, Kashiwabara H, Yokoyama T, Tanada S: Long-term assessment of posttransplant renal prognosis with 31 P magnetic resonance spectroscopy. Transplantation 72: 627–630, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Vyhnanovská P, Dezortová M, Herynek V, Táborský P, Viklický O, Hájek M: In vivo 31P MR spectroscopy of human kidney grafts using the 2D-chemical shift imaging method. Transplant Proc 43: 1570–1575, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Rosenkrantz AB, Raj S, Babb JS, Chandarana H: Comparison of 3D two-point Dixon and standard 2D dual-echo breath-hold sequences for detection and quantification of fat content in renal angiomyolipoma. Eur J Radiol 81: 47–51, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Dixon WT: Simple proton spectroscopic imaging. Radiology 153: 189–194, 1984 [DOI] [PubMed] [Google Scholar]
- 50.Rinella ME, McCarthy R, Thakrar K, Finn JP, Rao SM, Koffron AJ, Abecassis M, Blei AT: Dual-echo, chemical shift gradient-echo magnetic resonance imaging to quantify hepatic steatosis: Implications for living liver donation. Liver Transpl 9: 851–856, 2003 [DOI] [PubMed] [Google Scholar]