Summary
Background and objectives
An open-label, multicenter, randomized phase II trial was conducted from July 1, 2005 to March 29, 2011 to compare two protocols for treating children with frequently relapsing nephrotic syndrome using microemulsified cyclosporine.
Design, setting, participants, & measurements
Ninety-three children with frequently relapsing nephrotic syndrome were randomly assigned to group A (n=46) or group B (n=47). In both groups, the 2-hour postdose cyclosporine level was monitored. For group A, the cyclosporine target was set to 600–700 ng/ml for the first 6 months and 450–550 ng/ml for the next 18 months; for group B, it was set to 450–550 ng/ml for the first 6 months and 300–400 ng/ml for the next 18 months. The primary end point was the sustained remission rate. At the end of the study, if there was no difference in safety profile between the two groups and the sustained remission rate in group A was superior to group B with a decision threshold of 8%, then the regimen for group A would be determined the better treatment.
Results
Eight children from an ineligible institution, where cyclosporine levels were not measured, were excluded from all analyses. At 24 months, the sustained remission rate was nonsignificantly higher in group A (n=43) than group B (n=42; 64.4% versus 50.0%; hazard ratio, 0.57; 95% confidence interval, 0.29 to 1.11; P=0.09), and the progression-free survival rate was significantly higher (88.1% versus 68.4%; hazard ratio, 0.33; 95% confidence interval, 0.12 to 0.94; P=0.03). The relapse rate was significantly lower in group A than group B (0.41 versus 0.95 times/person-year; hazard ratio, 0.43; 95% confidence interval, 0.19 to 0.84; P=0.02). The rate and severity of adverse events were similar in both treatment groups.
Conclusion
The sustained remission rate was not significantly different between the two treatment groups, but the regimen with the higher 2-hour postdose cyclosporine level target improved progression-free survival and reduced the relapse rate.
Introduction
Cyclosporine has been found to be effective for the treatment of frequently relapsing nephrotic syndrome (FRNS) or steroid-dependent nephrotic syndrome (SDNS) in children (1–6). Kidney Disease Improving Global Outcomes Clinical Practice Guideline for Glomerulonephritis recommends that cyclosporine or tacrolimus be given as corticosteroid-sparing agents for FRNS children (7). However, tacrolimus is still off label for FRNS in Japan. Therefore, the development of more effective and safer regimens with cyclosporine for FRNS children is important.
A protocol for treating children with FRNS using Sandimmune, an older formulation of cyclosporine, was previously established in Japan (8). In patients who received Sandimmune in a dose that maintained the whole-blood trough level (C0) at 80–100 ng/ml for the first 6 months and 60–80 ng/ml for the next 18 months, the estimated sustained remission rate (SRR) was 57% at month 24, and mild chronic cyclosporine nephrotoxicity was found in 20% of patients who underwent renal biopsy after 24 months of treatment.
In 2000, a newer formulation of microemulsified cyclosporine (mCyA; Neoral Novartis, Basel, Switzerland) was introduced in Japan. We previously examined whether treatment with mCyA, titrated by C0 monitoring with the C0 target set to the same concentrations mentioned above, was effective and safe in children with FRNS the Japanese Study Group of Renal Disease in Children 07 (the JSRDC07) trial (9). In the JSRDC07 trial, the estimated SRR at month 24 was 58.1%, and mild chronic cyclosporine nephrotoxicity was detected in only 8.6% of patients. Based on these results, the Japanese Society for Pediatric Nephrology (JSPN) recommended mCyA titrated by C0 monitoring, in which the C0 target was set to 80–100 ng/ml for the first 6 months and 60–80 ng/ml for the next 18 months, as the standard treatment with mCyA for children with FRNS.
Because cyclosporine is stably absorbed after administration of mCyA, the dose of mCyA can be titrated based on the area under the concentration time curve during the first 4 hours after treatment (AUC0–4) in children who receive kidney transplants (10). It has been reported that the best single-point predictor of AUC0–4 is the 2-hour postdose cyclosporine level (C2) and that C2 management of mCyA treatment is effective and safe in pediatric kidney transplant recipients (11). One of the clinical benefits of C2 monitoring, shown in the majority of studies on transplantation, is a reduction in mean cyclosporine dose, which may reduce the rate of adverse effects of cyclosporine, including chronic cyclosporine nephrotoxicity (12). Several reports described the efficacy and/or safety of mCyA treatment with C2 monitoring, mainly with single daily dose, in children with FRNS (13–20). However, there were few prospective studies to determine appropriate C2 target with two divided oral doses of mCyA in children with FRNS. In addition, it is not known whether C2 monitoring or C0 monitoring is better in children with FRNS.
To address these questions, we first needed to decide on an appropriate treatment protocol for C2 monitoring in children with FRNS. Therefore, we conducted an open-label, multicenter, randomized phase II controlled trial designed to select a better treatment for FRNS in children by comparing two target cyclosporine C2 levels (the Japanese Study Group of Kidney Disease in Children 03 [JSKDC03] trial; University Hospital Medical Information Network–Clinical Trials Registry: C000000008).
Materials and Methods
Patients
The study was approved by the institutional review board at each center and complied with the Declaration of Helsinki. Written assent was obtained from patients when they were old enough to understand, and written informed consent was obtained from all of their parents.
Patients were registered from 14 centers in Japan (Supplemental Table 1) and randomized to the higher (group A) or lower target C2 group (group B) between July 1, 2005 and January 9, 2009. To be included in the study, patients needed to (1) have FRNS, (2) be 1–18 years old, and (3) have renal biopsy findings showing minor glomerular abnormalities, diffuse mesangial proliferation, or FSGS within 12 months before enrollment. Patients were excluded from the study if they had been treated with cyclosporine, were pregnant, or had (1) a history of steroid resistance, (2) a creatinine clearance rate of ≤60 ml/min per 1.73 m2, (3) active infections, (4) secondary nephrotic syndrome, (5) poorly controlled hypertension, or (6) severe liver dysfunction. The last patient visit was on March 29, 2011.
The definitions of nephrotic syndrome (21,22) are as follows. Nephrotic syndrome was defined as urine protein-to-creatinine ratio≥1.8 or above and serum albumin≤2.5g/dl. Remission was defined as negative protein on urine dipstick test or urine protein-to-creatinine ratio<0.2 for 3 consecutive days. Relapse was defined as protein≥2+ on urine dipstick test for 3 consecutive days. FRNS was defined as two or more relapses within 6 months after initial remission or four or more relapses within any 12-month period. SDNS was defined as relapse occurring two times consecutively during the reduction of the prednisolone dosage or within 2 weeks after its discontinuation. Steroid-resistant nephrotic syndrome (SRNS) was defined as the daily administration of prednisolone at 60 mg/m2 per day that does not lead to remission within 4 weeks.
Trial Design
The JSKDC03 was an open-label, multicenter, prospective, randomized phase II controlled trial. We adopted the selection design proposed by Simon et al. (23) and generalized by Sargent et al. (24), which is frequently used for the development of antibacterial and anticancer agents, for the comparison of the C2 monitoring of mCyA in phase II trial setting. The selection design has been used to choose which regimen should be further tested in a phase III trial, typically in limited number of patients. Randomized phase II design does not bring a confirmatory result; however, it has the advantage of being able to evaluate with a uniform evaluation criteria.
The purpose of this trial was to select a better treatment for FRNS in children by comparing two target cyclosporine C2 levels: a higher target C2 (group A) and a lower target C2 (group B). A statistically significant difference in primary end point between the two groups was not required in this trial. The criteria for selection were as follows: when there was no difference in safety profile between the two groups and the SRR at 24 months in group A was superior to the SRR in group B with a decision threshold of 8%, the regimen for group A was selected as the better treatment for FRNS. Otherwise, the regimen for group B was selected. The decision threshold of 8% was set before the start of the study based on a consensus reached by pediatric nephrologists in the JSKDC.
The total sample size was determined as 100. Randomization of the patients into two groups was performed in a 1:1 ratio with a dynamic balancing method. A prestudy calculation of sample size and the method of randomization are described in detail in Supplemental Appendix.
Experimental Intervention
Within 7 days after randomization, treatment with mCyA commenced. mCyA was administered orally at least 15 minutes before meals and started at a dose of 3–4 mg/kg body wt divided into two equal doses. We adjusted each dose of mCyA to the target C2 ranges by increasing or decreasing it by 20%–30%.
The total duration of mCyA treatment was 24 months. Group A received mCyA in a dose producing a whole-blood C2 level between 600 and 700 ng/ml for the first 6 months and between 450 and 550 ng/ml for the next 18 months. Group B received mCyA in a dose producing a whole-blood C2 level between 450 and 550 ng/ml for the first 6 months and between 300 and 400 ng/ml for the next 18 months.
How to determine the target C2 levels and corticosteroid treatment at the relapse during the study is described in Supplemental Appendix. No patients received corticosteroids as a maintenance therapy. Measurement of cyclosporine concentrations and other variables is also described in Supplemental Appendix.
After 24 months of treatment, the dose of mCyA was tapered off within 3 months, and all patients were scheduled to undergo renal biopsies.
The use of immunosuppressive agents, except for prednisolone and mCyA, was prohibited during the trial. The experimental intervention was stopped if (1) patients developed FRNS, SDNS, or SRNS after the start of mCyA treatment, (2) patients and/or their parents required the intervention to be stopped, (3) patients developed severe adverse events that required intervention to be stopped, (4) the primary investigator or the institutional review board at each center decided to stop the trial, or (5) patients were not followed up.
End Points
The primary end point was relapse-free survival based on the period of time until the first relapse. There were two secondary end points. One end point was the probability of progression-free survival based on the time until the progression to FRNS, SDNS, or SRNS. The other end point was the relapse rate, which was calculated by dividing the total number of relapses by the total duration of observations for all patients combined.
We also evaluated the rate and severity of development of chronic cyclosporine nephrotoxicity and other adverse events that occurred during the trial. A pathologist on our team (M.N.) evaluated the development of chronic cyclosporine nephrotoxicity, which was defined as cyclosporine-associated arteriolopathy and/or cyclosporine-induced tubulointerstitial lesions showing characteristic striped tubulointerstitial lesions.
Statistical Analyses
Statistical analyses were performed on an intention-to-treat basis. Individuals that did not complete 24 months of the study were still included in the analysis and counted as events. The Kaplan–Meier method was used to estimate the SRR at 24 months after randomization based on the relapse-free survival. The Cox proportional hazard model was used to estimate the hazard ratio and its 95% confidence interval (95% CI) between the groups. These methods and the log-rank test were also used to analyze progression-free survival. The unequal variance t test was used to compare the distributions of the average of C2 and AUC0–4. Fisher’s exact test was used to assess the statistical significance of comparisons at the patient level. All statistical analyses were conducted using SAS 9.1 software (SAS Institute, Cary, NC).
Adverse events corresponding to defined classes were tabulated first for 2 years.
Results
Patients
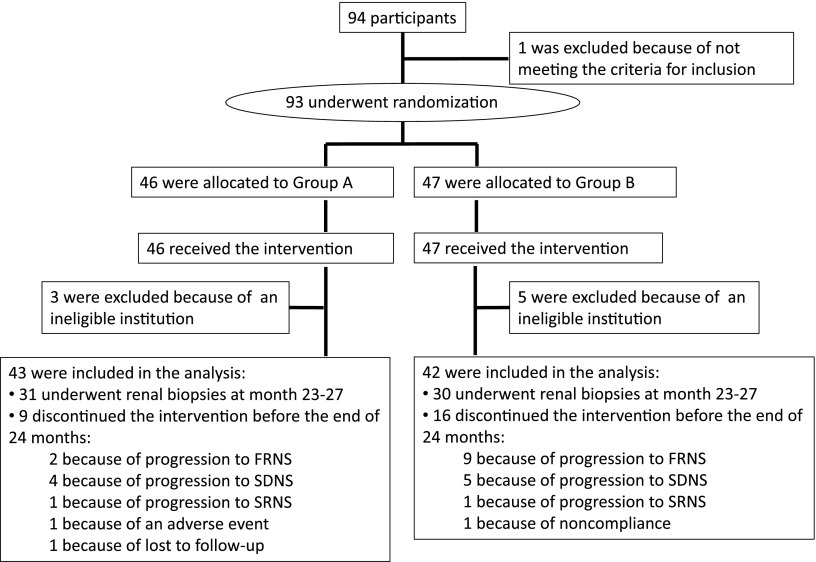
Between April of 2005 and March of 2009, 94 children with minimal change nephrotic syndrome, diagnosed based on pathologic analysis, were registered. One patient was later found to be ineligible because of not meeting the definition of FRNS; therefore, 93 patients were randomly assigned to two treatment groups (group A, n=46; group B, n=47). However, eight patients (three patients in group A; five patients in group B) were from an institution deemed ineligible, because C2 levels were not measured; thus, these patients were excluded from all analyses. Twenty-five patients discontinued the treatment regimen before the end of the 2-year study period but were included in the analysis for their time in the study. Eleven patients (two patients in group A; nine patients in group B) discontinued treatment because of progression to FRNS. Nine patients (four patients in group A; five patients in group B) discontinued treatment because of progression to SDNS. Two patients (one patient in group A; one patient in group B) discontinued treatment because of progression to SRNS. One patient (group A) discontinued treatment because of an adverse event, one patient (group A) discontinued treatment because of loss to follow-up, and one patient (group B) discontinued treatment because of noncompliance (Figure 1).
Figure 1.
Flow diagram of the patients. FRNS, frequently relapsing nephrotic syndrome; SDNS, steroid-dependent nephrotic syndrome; SRNS, steroid-resistant nephrotic syndrome.
Characteristics of the patients are shown in Table 1. There was no clinically important difference between the two treatment groups.
Table 1.
Characteristics of the patients
| Variables | Group A (n=43) | Group B (n=42) |
|---|---|---|
| Men | 32 (74.4) | 31 (73.8) |
| Age at entry (yr) | 7.0±4.3 | 7.1±3.7 |
| 1–5 | 25 (59.5) | 19 (45.2) |
| 6–10 | 8 (19.1) | 14 (33.3) |
| 11–13 | 6 (14.3) | 5 (11.9) |
| 14–18 | 4 (9.3) | 4 (9.5) |
| Minimal change subtype of NS | 43 (100.0) | 42 (100.0) |
| Duration of NS (mo) | 18.9±35.5 | 12.7±15.9 |
| History of SDNS | 26 (60.5) | 26 (61.9) |
| Previous treatment with immunosuppressive agent(s) | 8 (18.6) | 10 (23.8) |
| Mizoribine | 6 (14.0) | 9 (21.4) |
| Cyclophosphamide | 1 (2.3) | 1 (2.4) |
| Chlorambucil | 1 (2.3) | 0 (0) |
| Total protein (g/dl) | 5.9±0.6 | 5.8±0.7 |
| Albumin (g/dl) | 3.4±0.7 | 3.3±0.7 |
| BUN (mg/dl) | 11.5±4.0 | 12.8±3.4 |
| Creatinine (mg/dl) | 0.3±0.1 | 0.4±0.1 |
| Study baseline eGFR (ml/min per 1.73 m2) | 122.3±30.6 | 116.5±21.4 |
Values are n (%) or mean±SD. NS, nephrotic syndrome; SDNS, steroid-dependent nephrotic syndrome; eGFR, estimated GFR.
C2 and AUC0–4 Levels of Cyclosporine
The mean C2 levels during the first 6 months, the mean C2 levels during the next 18 months, and the AUC0–4 levels at 3 and 9 months after randomization were all significantly higher in group A than group B (P<0.001 in all cases) (Table 2). The distribution of exact mean C2 levels and actual doses of mCyA received by patients in the two groups are shown in Supplemental Tables 2 and 3, respectively.
Table 2.
Mean 2-hour postdose cyclosporine levels and areas under the concentration time curve during the first 4 hours after treatment with cyclosporine
| Cyclosporine | Group A (Mean±SD) | Group B (Mean±SD) | P Value |
|---|---|---|---|
| C2 (ng/ml) | |||
| Months 1–6 | 566.4±86.9 (n=43) | 472.7±73.7 (n=42) | <0.001 |
| Months 7–24 | 489.5±56.4 (n=40) | 382.2±86.8 (n=37) | <0.001 |
| AUC0–4 (ng⋅h/ml) | |||
| Month 3 | 1944.7±487.9 (n=39) | 1554.7±462.8 (n=40) | <0.001 |
| Month 9 | 1704.7±545.2 (n=36) | 1316.6±366.0 (n=34) | <0.001 |
C2, 2-hour postdose cyclosporine level; AUC0–4, area under the concentration time curve during the first 4 hours after treatment with cyclosporine.
Efficacy
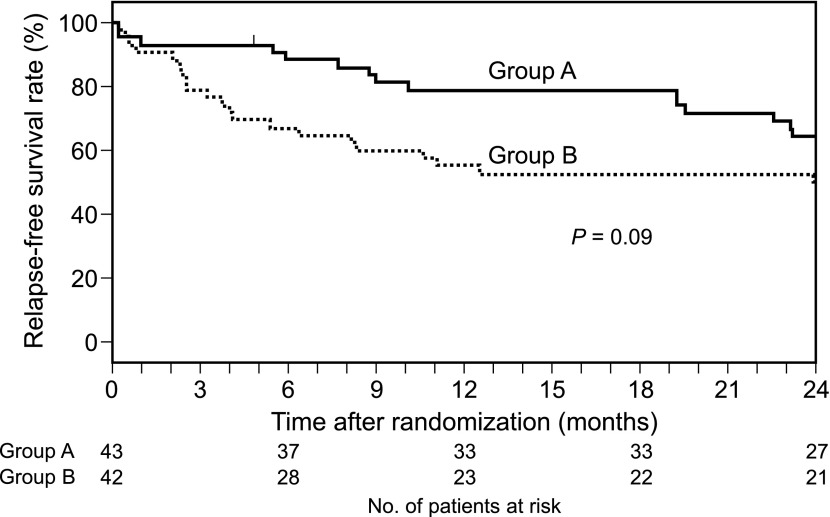
The primary end point, relapse-free survival, is shown in Figure 2. The estimated SRR 24 months after randomization was 64.4% (95% CI, 48.0% to 76.8%) in group A and 50.0% (95% CI, 34.2% to 63.9%) in group B. The SRR in group A was 14.4% higher than the SRR in group B, which was larger than the decision threshold of 8%; 27 of 43 patients in group A and 21 of 42 patients in group B had not experienced any relapse by the end of 24 months after randomization. The hazard ratio for relapse was 0.57 (95% CI, 0.29 to 1.11; P=0.09). The relapse rates in groups A and B were 0.41 and 0.95/person-year, respectively. The ratio of the two relapse rates was 0.43 (95% CI, 0.19 to 0.84; P=0.02) (Table 3).
Figure 2.
Relapse-free survival probability (Kaplan–Meier curves).
Table 3.
Relapse rates
| Treatment Group | Total Number of Relapses | Duration of Observation (d) | Relapse Rate (per person-yr) | Ratio of Relapse Rates (95% Confidence Interval) | P Value |
|---|---|---|---|---|---|
| Group A | 34 | 30,259 | 0.41 | 0.43(0.19 to 0.84) | 0.0 |
| Group B | 66 | 25,490 | 0.95 |
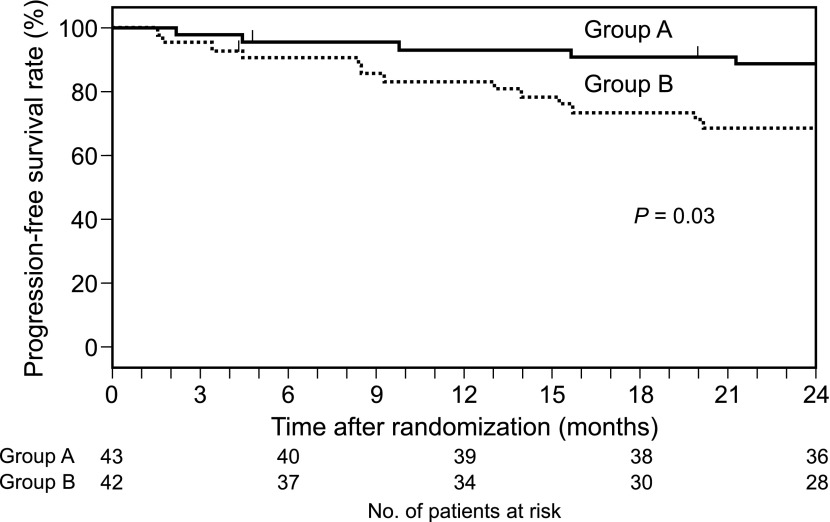
The estimated progression-free survival rate at 24 months was 88.1% in group A and 68.4% in group B; seven patients in group A showed progression (two patients to FRNS, four patients to SDNS, and one patient to SRNS), whereas 15 patients in group B showed progression (nine patients to FRNS, five patients to SDNS, and one patient to SRNS). The hazard ratio for progression was 0.33 (95% CI, 0.12 to 0.94; P=0.03) (Figure 3).
Figure 3.
Progression-free survival probability (Kaplan–Meier curves).
Safety
The medians (25th and 75th percentiles) of estimated GFRs before mCyA treatment and at month 24 were 119.0 (106.4–130.9) and 116.0 (106.9–129.0) in group A and 114.0 (102.4–125.0) and 121.3 (109.9–134.3) in group B, respectively. There was no difference between the two groups; 61 patients (31 patients in group A; 30 patients in group B) underwent renal biopsies: 60 patients during months 23–27 and one patient at month 31. Two patients in group A (6.5%) and zero patients in group B developed mild to moderate chronic cyclosporine nephrotoxicity (Supplemental Table 4). This difference in the rate of development of chronic cyclosporine nephrotoxicity was not statistically significant.
A summary of other adverse events reported during the trial is shown in Table 4. We report cumulative events that occurred within 24 months after randomization, because this time point is when all participants had had an equal opportunity to have an event. The rate and severity of adverse events were similar in both treatment groups. Three patients in group A and two patients in group B had grade III adverse events requiring hospitalization, including one patient in group A who discontinued protocol treatment because of posterior reversible leukoencephalopathy syndrome (25) (month 20), which recovered completely after discontinuation of the protocol treatment. Two of the patients in group A and both of the patients in group B subsequently recovered and restarted protocol treatment as recommended by a physician (Table 4).
Table 4.
Summary of adverse events that occurred within 24 months after randomization
| Event | Group A (n=43) n (%) | Group B (n=42) n (%) |
|---|---|---|
| Grade 3 adverse events | ||
| Pneumoniaa | 3b,c,d (7.0) | 1e (2.4) |
| Encephalopathya | 1c (2.3) | 1f (2.4) |
| Posterior reversible encephalopathy syndromea | 1b (2.3) | 0 |
| Pneumomediastinumg | 1c (2.3) | 0 |
| Grade 1 or 2 adverse events | ||
| Infectiona | 15 (34.9) | 13 (31.0) |
| Asthmaa | 3 (7.0) | 1 (2.4) |
| Edemaa | 1 (2.3) | 2 (4.8) |
| Moon facea | 3 (7.0) | 4 (9.5) |
| Centripetal obesitya | 2 (4.7) | 1 (2.4) |
| Hypertrichosisa | 23 (53.5) | 20 (47.6) |
| Acnea | 4 (9.3) | 2 (4.8) |
| Cutaneous striaea | 0 | 1 (2.4) |
| Hypertensiong | 7 (16.3) | 5 (11.9) |
| Gingival hyperplasiag | 4 (9.3) | 7 (16.7) |
| Gastrointestinal eventg | 2 (4.7) | 0 |
| Dermatological eventg | 5 (11.6) | 3 (7.1) |
| Neuropsychiatric eventg | 4 (9.3) | 3 (7.1) |
| Paing | 0 | 3 (7.1) |
| Cataractg | 2 (4.7) | 0 |
| Glaucomag | 1 (2.3) | 0 |
| Chronic sinusitisg | 0 | 1 (2.4) |
| Coughg | 1 (2.3) | 0 |
| Hyperglycemiag | 2 (4.7) | 2 (4.8) |
| Hyperkalemiag | 1 (2.3) | 1 (2.4) |
| Hyperbilirubinemiag | 2 (4.7) | 3 (7.1) |
| Hyperuricemiag | 1 (2.3) | 1 (2.4) |
| High-serum glutamic oxaloacetic transaminaseg | 1 (2.3) | 3 (7.1) |
| High-serum glutamic pyruvic transaminaseg | 2 (4.7) | 1 (2.4) |
| High amylaseg | 1 (2.3) | 0 |
| High serum creatinine phosphokinaseg | 1 (2.3) | 0 |
| Low GFRg | 1 (2.3) | 0 |
| Othersg | 1 (2.3) | 3 (7.1) |
Multiple reports were recorded for these adverse events.
One patient in group A had pneumonia at month 11 and recovered after 7 days without discontinuing protocol treatment. The same patient had posterior reversible encephalopathy syndrome at month 20, and protocol treatment was discontinued. He recovered completely after 10 days.
One patient in group A had pneumonia, encephalopathy, and pneumomediastinum after influenza infection at month 5 and recovered after 7 days. Protocol treatment was restarted after the recovery.
One patient in group A had pneumonia at month 21 and recovered after 12 days without discontinuing protocol treatment.
One patient in group B had pneumonia at month 5 and recovered after 7 days without discontinuing protocol treatment.
One patient in group B had encephalopathy after rotavirus infection at month 1 and recovered after 7 days. Protocol treatment was restarted after the recovery.
Only the first occurrence of these adverse events was recorded.
Discussion
This study is the first to attempt to select better C2 levels of cyclosporine in the form of mCyA for FRNS in children. The SRR in group A was 14.4% higher than the SRR in group B, which was larger than the decision threshold of 8%. Also, there was no difference between the two groups with respect to the frequency and severity of adverse events. Therefore, we considered that the C2 monitoring regimen for group A, in which the target C2 level was 600–700 ng/ml for the first 6 months and 450–550 ng/ml for the next 18 months, was better than the regimen for group B, in which the target C2 level was 450–550 ng/ml for the first 6 months and 300–400 ng/ml for the next 18 months. Referencing the report by Ushijima et al. (26) on the pharmacokinetic profile of Japanese nephrotic syndrome children treated with mCyA, the mean C0 levels for months 7–24 in group A might have ranged from 60 to 80 ng/ml, which was lower than the levels in the previous studies (7).
We found that the rate of relapse of nephrotic syndrome was significantly lower in group A than group B patients. This finding agrees with a previous finding that FRNS patients with higher C2 levels at month 1 tend to have lower relapse rates during cyclosporine treatment (9).
In the previous studies of mCyA treatment by C2 monitoring for childhood FRNS, the mean relapse rates varied from 0.2 to 1.5 per year under the mean C2 levels, which ranged from 497.8 to 729.0 ng/ml (13,16,18,20). The relapse rate in group A in the present study (0.41/person-year) was not inferior to the relapse rates in previous studies. Therefore, we considered that the regimen with C2 target for group A is acceptable for the treatment for childhood FRNS. However, it remains to be elucidated whether the regimen is also acceptable for other populations, because most of C2 monitoring studies for childhood FRNS were carried out in Japan.
Several grade III adverse events were reported in both groups in this trial. However, all patients with those severe adverse events recovered completely, and most patients restarted protocol treatment. Therefore, we considered adverse events in this trial acceptable. In the present study, two patients (4.7%) in group A developed mild to moderate chronic cyclosporine nephrotoxicity, and zero patients in group B developed this condition. Although the reason is unclear, the prevalence of chronic cyclosporine nephrotoxicity in the present study was much lower than the prevalence in a previous study (discussed in Supplemental Appendix) (15), suggesting that the regimens used in the present study were safe with respect to the development of this condition. The two patients who developed cyclosporine nephrotoxicity both had 9-month AUC levels that seemed to be notably higher than the mean for group A (Supplemental Table 4). However, it is premature to make a conclusion that the higher 9-month AUC levels were responsible for the nephrotoxicity, because the number of patients who developed chronic cyclosporine nephrotoxicity was very low.
One limitation of our study is that, at one particular center, C2 levels were not measured in most patients. Because we had defined the full analysis set as registered patients whose treatments were correctly started in the protocol, the steering committee considered that center to be ineligible and decided that all eight patients at the center should be excluded from the full analysis set.
Another limitation is that the mean C2 levels during the first 6 months in group A did not reach the target range, suggesting that it is difficult to control C2 levels in children, especially when the C2 target is relatively high. We speculate that a slight difference in dose of mCyA may induce a relatively large difference in C2 concentrations in children when the C2 target is relatively high. Nevertheless, the mean C2 levels in group A were significantly higher than the mean C2 levels in group B throughout the trial. In addition, the levels of AUC0–4 at months 3 and 9 were significantly higher in group A than group B. We, therefore, conclude that patients in both groups were treated in accordance with the protocol. Additional discussion on the target C2 levels for phase III trials is in Supplemental Appendix.
It is still controversial whether C2 or C0 monitoring is better for renal transplant recipients (10,11,27–34). It is also unclear whether C2 or C0 monitoring is better for children with FRNS treated with mCyA. Although our study shows that C2 monitoring with the target C2 set for group A is promising, phase III trials are required to compare the efficacy and safety of the regimen with the efficacy and safety of the JSPN-recommended C0 monitoring protocol.
Disclosures
K. Iijima received grants from Pfizer Japan, Inc.; Kyowa Hakko Kirion, Co. Ltd.; Abbot Japan Co. Ltd.; Takeda Pharmaceutical Co. Ltd.; Asahi Kasei Pharma Corporation; Astellas Pharma, Inc.; Terumo Medical Care K.K.; Chugai Pharmaceutical Co. Ltd.; Benesis (currently, Japan Blood Product Organization); Dainippon Sumitomo Pharma Co. Ltd.; Genzyme Japan K.K.; Novartis Pharma K.K.; Mizutori Clinic; AbbVie LLC; and Janssen Pharmaceutical K.K.; and lecture fees from Novartis Pharma K.K.; Asahi Kasei Pharma Corporation; Baxter Limited; Sanofi K.K.; Pfizer Japan, Inc.; Meiji Seika Pharma Co. Ltd.; Taisho Toyama Pharmaceutical Co. Ltd.; Kyorin Pharmaceutical Co. Ltd.; Kyowa Hakko Kirion, Co. Ltd.; Dainippon Sumitomo Pharma Co. Ltd.; Astellas Pharma, Inc.; Chugai Pharmaceutical Co. Ltd.; and Kowa Pharmaceutical Co. Ltd. S.I. received lecture fees from Asahi Kasei Pharma Corporation, Novartis Pharma K.K., and Chugai Pharmaceutical Co. Ltd. R.T. received a lecture fee from Pfizer Japan, Inc. K. Ishikura received a lecture fee from Novartis Pharma K.K. Y. Ohashi received a lecture fee from Chugai Pharmaceutical Co. Ltd. N.Y. received grants from Astellas Pharma, Inc.; Dainippon Sumitomo Pharma Co. Ltd.; Teijin Pharma Limited; Bayer Yakuhin Ltd; Novartis Pharma K.K.; Kyowa Hakko Kirin Co. Ltd.; Benesis; GlaxoSmithKline K.K.; Asahi Kasei Pharma Corporation; Kyorin Pharmaceutical Company Limited; Otsuka Pharmaceutical Co. Ltd.; Nippon Shinyaku Co. Ltd.; Japan Blood Products Organization; and Maruho Co. Ltd.; and lecture fees from Novartis Pharma K.K.; Dainippon Sumitomo Pharma Co. Ltd.; Kyorin Pharmaceutical Company Limited; Asahi Kasei Pharma Corporation; Astellas Pharma, Inc.; Chugai Pharmaceutical Co. Ltd.; Merck & Co., Inc.; Boehringer Ingelheim; Ono Pharmaceutical Co. Ltd; and Genzyme Japan K.K. The other authors have no conflicts of interest to declare.
Supplementary Material
Acknowledgments
We thank all our patients and their families who participated in the Japanese Study Group of Kidney Disease in Children 03.
This study was supported by Ministry of Health, Labour and Welfare, Japan Grant H15-shouni-002.
This study was partly presented at Kidney Week 2011 on November 12, 2011, in Philadelphia, PA, and published in part in abstract form (Iijima et al., J Am Soc Nephrol 22: 6B, 2011).
Physicians who participated in the Japanese Study Group of Kidney Disease in Children 03 are listed in Supplemental Appendix.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13071212/-/DCSupplemental.
References
- 1.Eddy AA, Symons JM: Nephrotic syndrome in childhood. Lancet 362: 629–639, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Inoue Y, Iijima K, Nakamura H, Yoshikawa N: Two-year cyclosporin treatment in children with steroid-dependent nephrotic syndrome. Pediatr Nephrol 13: 33–38, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Tanaka R, Yoshikawa N, Kitano Y, Ito H, Nakamura H: Long-term ciclosporin treatment in children with steroid-dependent nephrotic syndrome. Pediatr Nephrol 7: 249–252, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Kitano Y, Yoshikawa N, Tanaka R, Nakamura H, Ninomiya M, Ito H: Ciclosporin treatment in children with steroid-dependent nephrotic syndrome. Pediatr Nephrol 4: 474–477, 1990 [DOI] [PubMed] [Google Scholar]
- 5.El-Husseini A, El-Basuony F, Mahmoud I, Sheashaa H, Sabry A, Hassan R, Taha N, Hassan N, Sayed-Ahmad N, Sobh M: Long-term effects of cyclosporine in children with idiopathic nephrotic syndrome: A single-centre experience. Nephrol Dial Transplant 20: 2433–2438, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Cattran DC, Alexopoulos E, Heering P, Hoyer PF, Johnston A, Meyrier A, Ponticelli C, Saito T, Choukroun G, Nachman P, Praga M, Yoshikawa N: Cyclosporin in idiopathic glomerular disease associated with the nephrotic syndrome: Workshop recommendations. Kidney Int 72: 1429–1447, 2007 [DOI] [PubMed] [Google Scholar]
- 7.KDIGO Clinical Practice Guideline for Glomerulonephritis : Steroid-sensitive nephrotic syndrome in children. Kidney Int Suppl 2: 163–171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishikura K, Ikeda M, Hattori S, Yoshikawa N, Sasaki S, Iijima K, Nakanishi K, Yata N, Honda M: Effective and safe treatment with cyclosporine in nephrotic children: A prospective, randomized multicenter trial. Kidney Int 73: 1167–1173, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Ishikura K, Yoshikawa N, Hattori S, Sasaki S, Iijima K, Nakanishi K, Matsuyama T, Yata N, Ando T, Honda M, Japanese Study Group of Renal Disease in Children : Treatment with microemulsified cyclosporine in children with frequently relapsing nephrotic syndrome. Nephrol Dial Transplant 25: 3956–3962, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Weber LT, Armstrong VW, Shipkova M, Feneberg R, Wiesel M, Mehls O, Zimmerhackl LB, Oellerich M, Tönshoff B, Members of the German Study Group on Pediatric Renal Transplantion : Cyclosporin A absorption profiles in pediatric renal transplant recipients predict the risk of acute rejection. Ther Drug Monit 26: 415–424, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Ferraresso M, Ghio L, Zacchello G, Murer L, Ginevri F, Perfumo F, Zanon GF, Fontana I, Amore A, Edefonti A, Vigano S, Cardillo M, Scalamogna M: Pharmacokinetic of cyclosporine microemulsion in pediatric kidney recipients receiving A quadruple immunosuppressive regimen: The value of C2 blood levels. Transplantation 79:1164–1168, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Knight SR, Morris PJ: The clinical benefits of cyclosporine C2-level monitoring: A systematic review. Transplantation 83: 1525–1535, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Fujinaga S, Hirano D, Murakami H, Ohtomo Y, Shimizu T, Kaneko K: Nephrotoxicity of once-daily cyclosporine A in minimal change nephrotic syndrome. Pediatr Nephrol 27: 671–674, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Griveas I, Visvardis G, Papadopoulou D, Nakopolou L, Karanikas E, Gogos K, Stavianoudakis G: Effect of cyclosporine therapy with low doses of corticosteroids on idiopathic nephrotic syndrome. Artif Organs 34: 234–237, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Kengne-Wafo S, Massella L, Diomedi-Camassei F, Gianviti A, Vivarelli M, Greco M, Stringini GR, Emma F: Risk factors for cyclosporin A nephrotoxicity in children with steroid-dependant nephrotic syndrome. Clin J Am Soc Nephrol 4: 1409–1416, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujinaga S, Ohtomo Y, Someya T, Shimizu T, Yamashiro Y, Kaneko K: Is single-daily low-dose cyclosporine therapy really effective in children with idiopathic frequent-relapsing nephrotic syndrome? Clin Nephrol 69: 84–89, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Nakahata T, Tanaka H, Tsugawa K, Kudo M, Suzuki K, Ito E, Waga S: C1-C2 point monitoring of low-dose cyclosporin a given as a single daily dose in children with steroid-dependent relapsing nephrotic syndrome. Clin Nephrol 64: 258–263, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Fujinaga S, Kaneko K, Takada M, Ohtomo Y, Akashi S, Yamashiro Y: Preprandial C2 monitoring of cyclosporine treatment in children with nephrotic syndrome. Pediatr Nephrol 20: 1359–1360, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Tanaka H, Tsugawa K, Suzuki K, Ito E: Renal biopsy findings in children receiving long-term treatment with cyclosporine a given as a single daily dose. Tohoku J Exp Med 209: 191–196, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Suzuki K, Oki E, Tsuruga K, Aizawa-Yashiro T, Ito E, Tanaka H: Benefits of once-daily administration of cyclosporine a for children with steroid-dependent, relapsing nephrotic syndrome. Tohoku J Exp Med 220: 183–186, 2010 [DOI] [PubMed] [Google Scholar]
- 21.International Study of Kidney Disease in Children : The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr 98: 561–564, 1981 [DOI] [PubMed] [Google Scholar]
- 22.International Study of Kidney Disease in Children : Early identification of frequent relapsers among children with minimal change nephrotic syndrome. A report of the International Study of Kidney Disease in Children. J Pediatr 101: 514–518, 1982 [DOI] [PubMed] [Google Scholar]
- 23.Simon R, Wittes RE, Ellenberg SS: Randomized phase II clinical trials. Cancer Treat Rep 69: 1375–1381, 1985 [PubMed] [Google Scholar]
- 24.Sargent DJ, Goldberg RM: A flexible design for multiple armed screening trials. Stat Med 20: 1051–1060, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Ishikura K, Ikeda M, Hamasaki Y, Hataya H, Nishimura G, Hiramoto R, Honda M: Nephrotic state as a risk factor for developing posterior reversible encephalopathy syndrome in paediatric patients with nephrotic syndrome. Nephrol Dial Transplant 23: 2531–2536, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Ushijima K, Uemura O, Yamada T: Age effect on whole blood cyclosporine concentrations following oral administration in children with nephrotic syndrome. Eur J Pediatr 171: 663–668, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Pescovitz MD, Barbeito R, Simulect US01 Study Group : Two-hour post-dose cyclosporine level is a better predictor than trough level of acute rejection of renal allografts. Clin Transplant 16: 378–382, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Cole E, Maham N, Cardella C, Cattran D, Fenton S, Hamel J, O’Grady C, Smith R: Clinical benefits of neoral C2 monitoring in the long-term management of renal transplant recipients. Transplantation 75: 2086–2090, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Trompeter R, Fitzpatrick M, Hutchinson C, Johnston A: Longitudinal evaluation of the pharmacokinetics of cyclosporin microemulsion (Neoral) in pediatric renal transplant recipients and assessment of C2 level as a marker for absorption. Pediatr Transplant 7: 282–288, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Mahalati K, Belitsky P, West K, Kiberd B, Fraser A, Sketris I, Macdonald AS, McAlister V, Lawen J: Approaching the therapeutic window for cyclosporine in kidney transplantation: A prospective study. J Am Soc Nephrol 12: 828–833, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Clase CM, Mahalati K, Kiberd BA, Lawen JG, West KA, Fraser AD, Belitsky P: Adequate early cyclosporin exposure is critical to prevent renal allograft rejection: Patients monitored by absorption profiling. Am J Transplant 2: 789–795, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Moore J, Tan K, Cockwell P, Krishnan H, McPake D, Ready A, Mellor S, Hamsho A, Ball S, Lipkin G, Borrows R: Risk factors for acute rejection in renal transplant recipients experiencing delayed graft function. Clin Transplant 22: 634–638, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Einecke G, Schütz M, Mai I, Fritsche L, Giessing M, Glander P, Neumayer HH, Budde K: Limitations of C2 monitoring in renal transplant recipients. Nephrol Dial Transplant 20: 1463–1470, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Kyllönen LE, Salmela KT: Early cyclosporine C0 and C2 monitoring in de novo kidney transplant patients: A prospective randomized single-center pilot study. Transplantation 81: 1010–1015, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.