Fig. 1.
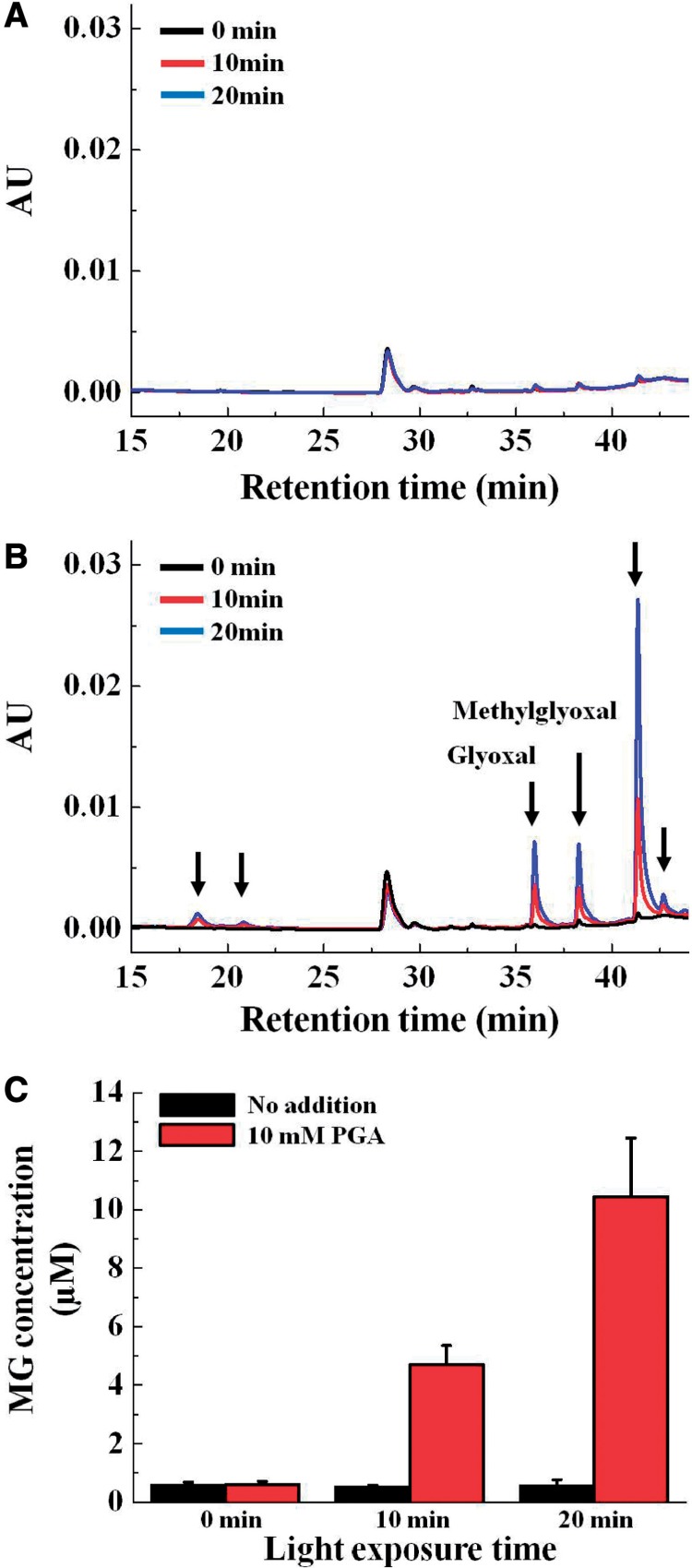
Chromatograms of o-phenylenediamine (OPD) derivatives of sugar-derived reactive carbonyls (RCs). 3-Phosphoglycerate (3-PGA) was absent (A) and present (B) in the reaction mixtures. Reaction mixtures (1 ml) that contained chloroplasts (40 µg of Chl) in the absence and presence of 10 mM 3-PGA were illuminated in red light (>640 nm, 400 µmol photons m−2 s−1) at 25°C. Typical chromatograms of sugar-derived RCs are shown. AU indicates relative absorbance units at 312 nm. The three lines indicate different light exposure times (black, 0 min; red, 10 min; and blue, 20 min). Arrows indicate sugar-derived RCs, the levels of which increased in the presence of 3-PGA. Glyoxal (GLO) and methyglyoxal (MG) were identified from the retention times of the commercially purchased compounds. (C) MG was quantified at the indicated time after illumination in the absence (black bars) or presence (red bars) of 3-PGA. HPLC of sugar-derived RCs is described in the Materials and Methods section. Values are expressed as means ± standard deviations of three independent experiments.
