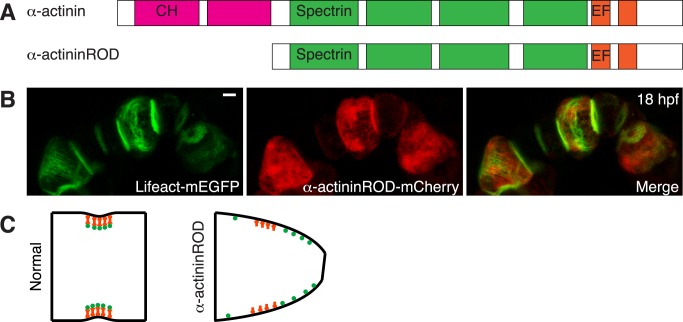
Figure 6. Role of α-actinin in notochord cell elongation.
(A) Structure of Ciona α-actinin and design of the α-actininROD mutant. Ciona α-actinin has two calponin homology domains (CH) at the carboxyl terminus, four spectrin-repeats (Spectrin) in the central region, and calmodulin-like (CaM) domain with two EF-hand motifs at the amino terminus. α-actininROD mutant lacks CH domains that have been shown to mediate actin binding. (B) Phenotype of α-actininROD–expressing notochord cells (maximal projection) at 18 hpf. Cells expressing the α-actininROD mutant have a wedged shape. Circumferential actin filaments are not restricted to the equatorial region. (C) A model of α-actinin (red) regulating the placement of actin filaments (green, showing cross-section) and cell elongation. In normal cells, α-actinin tethers actin filaments to the equatorial membrane through its actin- and membrane-binding activity. α-actininROD mutant fails to associate with actin filaments but nevertheless can occupy the equatorial membrane tether sites, therefore disrupting the anchoring of the actin filaments in the equatorial region, consequently resulting in mislocalized contraction. Scale bar, 5 µm.