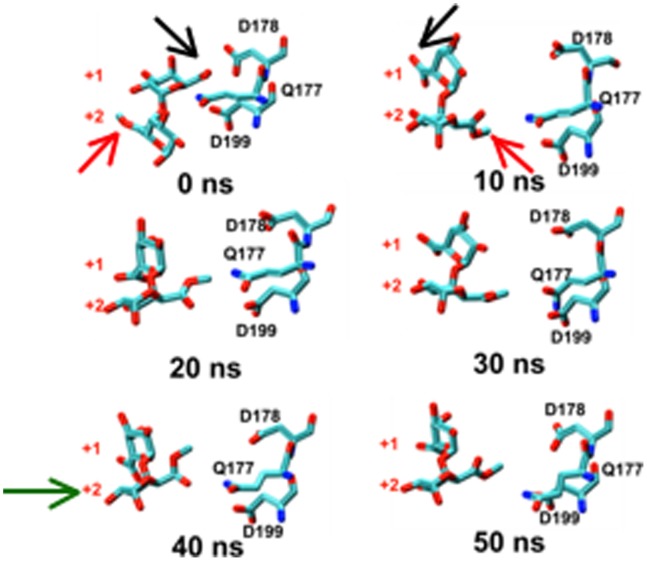
Figure 3. Rotation along the glycosidic bond and sliding of the substrate are the key events for the processive action of Ec-PME.
Time-evolution of the conformational variations occurring for the catalytic triad of Ec-PME and two monosaccharide residues of the HXM decasaccharide, labelled by the subsite in which they were originally docked (+1 or +2). 0–10 ns: concerted approximately 180° rotation of monosaccharide residues at subsites +1 and +2. 10–30 ns: Brownian fluctuations of this disaccharide moiety and active-site side chains. 30–50 ns: movement of disaccharide moiety relative to protein bringing, a fresh carboxymethyl group (that began in subsite +2 and is still labelled as such) into the active site at +1. Carbon atoms are colored cyan, oxygen atoms are colored red and nitrogen atoms are colored in blue. Black and red arrows show the carboxylate and carboxy-methylesterified groups of the monosaccharides initially docked in the +1 and +2 subsites respectively. The green arrow in the 40 ns window signals the movement of the monosaccharide that began in the +2 subsiste into the active site (+1 subsite). Note the similarity between the final position (50 ns) occupied by the sugar ring that began in subsite +2 and the initial position (0 ns) of the residue that started in subsite +1.