Abstract
Macrophages are critical proponents of the growth and development of pancreatic ductal adenocarcinoma. However, if properly instructed, macrophages can acquire anti-tumor properties, including the capacity to degrade the fibrous matrix that surrounds neoplastic lesions and to eliminate malignant cells, eventually leading to tumor regression.
Keywords: cancer, fibrosis, macrophage, PET imaging, metabolism, pancreas, tumoricidal
In pancreatic ductal adenocarcinoma (PDAC), reciprocal interactions between malignant cells and host cells including fibroblasts as well as inflammatory and vascular endothelial cells orchestrate a microenvironment that is immunosuppressive, fibrotic, and poorly vascular.1 This desmoplastic reaction that surrounds PDAC lesions constitutes a major obstacle to the efficacy of therapy.2 Indeed, cytotoxic drugs poorly penetrate this dense stromal matrix. Conversely, inflammatory cells, including macrophages, efficiently infiltrate PDAC lesions. Macrophages are the principal leukocyte infiltrating many solid malignancies. However, the presence of tumor-infiltrating macrophages most often portends a poor prognosis, mainly reflecting the ability of these cells to support oncogenesis and tumor progression (as they promote proliferation, invasion, and metastasis) and to inhibit adaptive immunosurveillance.3,4
To reverse the tumor-supporting phenotype of PDAC-infiltrating macrophages, we have targeted CD40, a member of the tumor necrosis factor receptor (TNFR) superfamily. CD40 is expressed on macrophages and other antigen-presenting cells (APCs), including B cells and dendritic cells, as well as some non-hematopoietic cells. CD40 agonist antibodies have been shown to “license” APCs for tumor-specific T-cell priming and activation.5 Preclinical studies also suggest that chemotherapy can synergize with CD40 agonists in the induction of productive tumor-specific T-cell immunity.6 Based on these data, we hypothesized that combining chemotherapy with a CD40 agonist in PDAC would reverse immunosuppression induced by macrophages and restore productive T-cell immunosurveillance. To test this hypothesis, we studied gemcitabine chemotherapy in combination with a CD40 agonist antibody in the KrasG12D/+;Trp53R172H/+;Pdx-1-Cre (KPC) mouse model of PDAC as well as in a Phase I clinical trial enrolling patients with advanced PDAC.7,8 This combinatorial regimen induced tumor regression in some KPC mice. Along similar lines, we observed major tumor regressions in some PDAC patients treated with gemcitabine in combination with a fully human CD40 agonist monoclonal antibody. In the KPC model, this therapeutic effect could be reproduced with a CD40 agonist alone, but not with gemcitabine alone, and was determined to be independent of T cells, which failed to infiltrate neoplastic lesions in response to therapy.
To understand the mechanism of tumor regression triggered by agonist CD40 therapy, we examined the tumor microenvironment of KPC mice receiving a CD40 agonist. In this setting, tumor-infiltrating macrophages were found to upregulate co-stimulatory molecules, consistent with a shift from a pro-tumor to an anti-tumor phenotype. To test the functional relevance of macrophages in the anti-tumor response induced with agonist CD40 therapy, we treated KPC mice with clodronate encapsulated liposomes (CELs), which deplete systemic, but not tumor-associated, macrophages. CELs abrogated the ability of CD40 agonist antibodies to induce tumor regression, pointing to a role for extratumoral macrophages as mediators of the anti-tumor immune response triggered by systemic CD40 activation. Consistent with this finding, tumor biopsies from 2 PDAC patients responding to gemcitabine combined with a CD40 agonist revealed a robust infiltrate of myeloid cells, in particular macrophages, coupled to an absence of T cells.
In our analysis of macrophages responding to agonist CD40 therapy, we observed a rapid disappearance of monocytes from the peripheral blood of both KPC mice and patients within 24 h after treatment. In KPC mice, we determined that monocytes responding to CD40 activation rapidly infiltrated neoplastic lesions, acquired tumoricidal properties, and facilitated the depletion of Type I collagen, a major component of the extracellular matrix (ECM) of the tumor microenvironment. However, such effects were limited to focal areas of neoplastic lesions, suggesting that some regions of the tumor are resistant to therapy. We used changes in glucose metabolism, as detected on [18F]-fluorodeoxyglucose (FDG)-based positron emission tomography/CT (PET/CT) imaging, as a surrogate marker of a therapeutic response to compare responses observed within primary and metastatic lesions in PDAC patients receiving a CD40 agonist in combination with chemotherapy. Using semi-automated imaging software, we quantified glucose metabolism within individual tumor lesions, within organs, and throughout the entire body of patients, in order to determine lesion, organ, and total body tumor burden. Our analysis revealed that primary pancreatic tumors tend to respond uniformly with each treatment cycle. Conversely, metastatic lesions varied in their response to treatment, with some lesions displaying a complete response and others within the same patient exhibiting no improvement with therapy. Treatment responses on FDG-PET/CT imaging, which were monitored within 2 weeks after the initiation of therapy, correlated with tumor responses detected on subsequent CT imaging and with improved overall survival.
Our findings suggest a role for macrophages in regulating the ECM that surrounds PDAC lesions. We believe that strategies designed to shift macrophages from stroma-supporting to stroma-degrading may enhance the delivery of chemotherapy to PDACs. In this way, macrophage-directed therapies may be effective at improving tumor debulking. However, long-term immunity will be necessary to prevent relapse/recurrence. In this regard, we found that CD40 agonist-based therapy was unable to restore productive T-cell immunosurveillance. This may reflect the presence of other immunosuppressive mediators or cell types, such as regulatory T cells, or a requirement for additional signals to shift macrophages toward fostering rather than suppressing T-cell immunity.
The heterogeneity in the treatment response that we observed with FDG-based imaging implies that some tumors are resistant to macrophage-based immunotherapy. This resistance mechanism is unknown but may reflect differences in various factors, including the propensity of specific PDAC lesions to attract myeloid cells, the amount and/or composition of tumor-associated ECM, or the sensitivity of malignant cells to the tumoricidal activity of macrophages.
In summary, we believe that macrophages are attractive targets for PDAC immunotherapy due to their tropism for desmoplastic tissues and their decisive impact on tumor biology. Macrophages can be induced to directly eliminate cancer cells and to degrade the stromal matrix that surrounds neoplastic lesions (Fig. 1). Strategies designed to harness the anti-tumor and anti-stroma potential of macrophages may improve the efficacy of existing anticancer therapies.
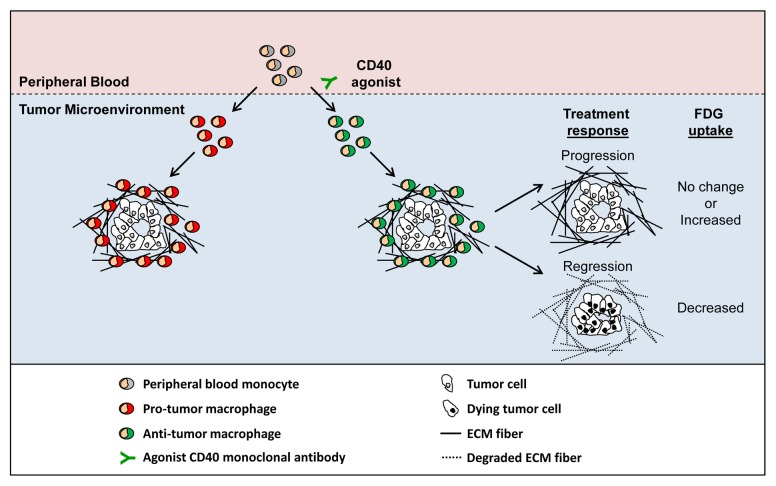
Figure 1. Re-directing the phenotype of tumor-infiltrating macrophages induces the regression of pancreatic adenocarcinoma lesions. Peripheral blood monocytes are routinely recruited to neoplastic lesions, where they can support tumor growth and development. The systemic administration of an agonist CD40 antibody shifts the phenotype of tumor-infiltrating macrophages from pro-tumor to anti-tumor. Anti-tumor macrophages induce the regression of some malignant lesions by eliminating cancer cells and degrading the extracellular matrix (ECM) that generally surrounds tumors. Tumor response is associated with changes in glucose metabolism as measured by [18F]-fluorodeoxyglucose (FDG) uptake detected on positron emission tomography/CT (PET/CT) imaging.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The author is supported by grants from the National Institutes of Health (K08 CA138907), W.W. Smith Charitable Trust Foundation, Department of Defense (W81XWH-12–1-0411), and the Damon Runyon Cancer Research Foundation for which Gregory L. Beatty is the Nadia's Gift Foundation Innovator of the Damon Runyon-Rachleff Innovation Award (DRR-15–12).
Glossary
Abbreviations:
- APC
antigen-presenting cell
- CEL
clodronate encapsulated liposome
- ECM
extracellular matrix
- FDG
[18F]-fluorodeoxyglucose
- KPC
KrasG12D/+,Trp53R172H/+,Pdx-1-Cre
- PDAC
pancreatic ductal adenocarcinoma
- PET/CT
positron emission tomography/computed tomography
Citation: Beatty GL. Macrophage immunotherapy for the treatment of pancreatic ductal adenocarcinoma. OncoImmunology 2013; 2:e26837; 10.4161/onci.26837
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26837
References
- 1.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–76. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–86. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–72. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63:4490–6. [PubMed] [Google Scholar]
- 7.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH, et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-1320. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]