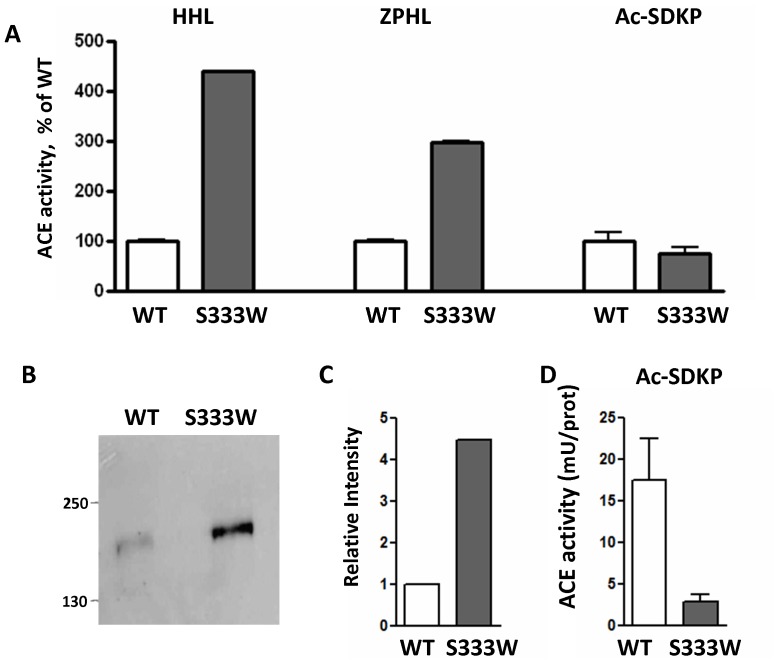
Figure 11. Ac-SDKP hydrolysis by recombinant mutant (S333W) ACE.
A. ACE activity of the membrane-bound form of ACE of CHO cells (lysates) stably expressing WT and mutant ACE was determined with substrates Hip-His-Leu and Z-Phe-His –Leu (as in Fig. 8) and with natural substrate Ac-SDKP (see Experimental Procedures). ACE activity (mU/ml of the lysate) was expressed as % of WT. B–C. The lysates of these cells (normalized by loading of 10 µg protein per lane) were subjected to SDS-PAGE (4-15% gradient gel) in reducing conditions for Western blotting (B) or protein quantification (C). B. Western blotting was performed with rat mAb 4G6 that recognizes the denatured epitope on the C-domain of mouse and human ACE [50]. Proteins transferred on PVDF-Plus membrane were revealed with 1/5 dilution of culture medium from hybridoma cells producing mAb 4G6. Molecular weight markers are shown by arrows on the left of panel B, which is a representative experiment. C. The relative amount of WT and mutant ACE revealed by Western blotting with mAb 4G6 (B) was quantified by the image analysis (densitometry) using ImageJ software (NIH). Data are expressed as mean ± SD of 2 independent measurements. D. ACE activity in the lysates of CHO cells stably transfected with WT and mutant ACE towards natural substrate Ac-SDKP 0.75 mM (A) was normalized to the amount of ACE protein in the lysate, determined by densitometry (C) after Western blotting (B).