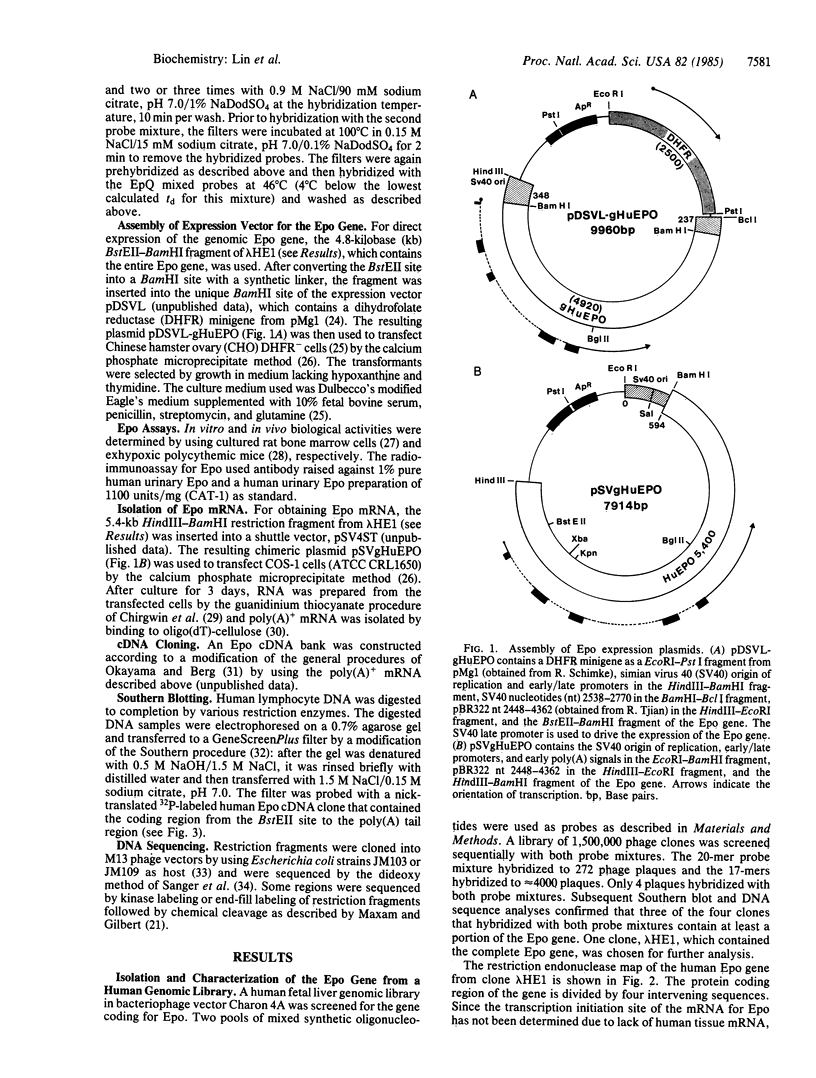
Abstract
The human erythropoietin gene has been isolated from a genomic phage library by using mixed 20-mer and 17-mer oligonucleotide probes. The entire coding region of the gene is contained in a 5.4-kilobase HindIII-BamHI fragment. The gene contains four intervening sequences (1562 base pairs) and five exons (582 base pairs). It encodes a 27-amino acid signal peptide and a 166-amino acid mature protein with a calculated Mr of 18,399. The erythropoietin gene, when introduced into Chinese hamster ovary cells, produces erythropoietin that is biologically active in vitro and in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostou A., Barone J., Kedo A., Fried W. Effect of erythropoietin therapy on the red cell volume of uraemic and non-uraemic rats. Br J Haematol. 1977 Sep;37(1):85–91. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. Plasma erythropoietin in chronic uraemia. Br Med J. 1965 Oct 30;2(5469):1036–1038. doi: 10.1136/bmj.2.5469.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTES P. M., BANGHAM D. R. Bio-assay of erythropoietin in mice made polycythaemic by exposure to air at a reduced pressure. Nature. 1961 Sep 9;191:1065–1067. doi: 10.1038/1911065a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cotes P. M. Immunoreactive erythropoietin in serum. I. Evidence for the validity of the assay method and the physiological relevance of estimates. Br J Haematol. 1982 Mar;50(3):427–438. doi: 10.1111/j.1365-2141.1982.tb01938.x. [DOI] [PubMed] [Google Scholar]
- Deininger P. L., Jolly D. J., Rubin C. M., Friedmann T., Schmid C. W. Base sequence studies of 300 nucleotide renatured repeated human DNA clones. J Mol Biol. 1981 Sep 5;151(1):17–33. doi: 10.1016/0022-2836(81)90219-9. [DOI] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Cochran M. D., Dobkin C., Reiser J., Weissmann C. Three regions upstream from the cap site are required for efficient and accurate transcription of the rabbit beta-globin gene in mouse 3T6 cells. Cell. 1983 Mar;32(3):695–706. doi: 10.1016/0092-8674(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERSLEV A. J. Physiologic control of red cell production. Blood. 1955 Sep;10(9):954–961. [PubMed] [Google Scholar]
- Erslev A. J., Caro J., Miller O., Silver R. Plasma erythropoietin in health and disease. Ann Clin Lab Sci. 1980 May-Jun;10(3):250–257. [PubMed] [Google Scholar]
- Eschbach J. W., Mladenovic J., Garcia J. F., Wahl P. W., Adamson J. W. The anemia of chronic renal failure in sheep. Response to erythropoietin-rich plasma in vivo. J Clin Invest. 1984 Aug;74(2):434–441. doi: 10.1172/JCI111439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried W. The liver as a source of extrarenal erythropoietin production. Blood. 1972 Nov;40(5):671–677. [PubMed] [Google Scholar]
- Fyhrquist F., Rosenlöf K., Grönhagen-Riska C., Hortling L., Tikkanen I. Is renin substrate an erythropoietin precursor? Nature. 1984 Apr 12;308(5960):649–652. doi: 10.1038/308649a0. [DOI] [PubMed] [Google Scholar]
- Garcia J. F., Ebbe S. N., Hollander L., Cutting H. O., Miller M. E., Cronkite E. P. Radioimmunoassay of erythropoietin: circulating levels in normal and polycythemic human beings. J Lab Clin Med. 1982 May;99(5):624–635. [PubMed] [Google Scholar]
- Gasser C. S., Simonsen C. C., Schilling J. W., Schimke R. T. Expression of abbreviated mouse dihydrofolate reductase genes in cultured hamster cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6522–6526. doi: 10.1073/pnas.79.21.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwasser E., Eliason J. F., Sikkema D. An assay for erythropoietin in vitro at the milliunit level. Endocrinology. 1975 Aug;97(2):315–323. doi: 10.1210/endo-97-2-315. [DOI] [PubMed] [Google Scholar]
- Goldwasser E. Erythropoietin and the differentiation of red blood cells. Fed Proc. 1975 Dec;34(13):2285–2292. [PubMed] [Google Scholar]
- Graber S. E., Krantz S. B. Erythropoietin and the control of red cell production. Annu Rev Med. 1978;29:51–66. doi: 10.1146/annurev.me.29.020178.000411. [DOI] [PubMed] [Google Scholar]
- JACOBSON L. O., GOLDWASSER E., FRIED W., PLZAK L. F. Studies on erythropoiesis. VII. The role of the kidney in the production of erythropoietin. Trans Assoc Am Physicians. 1957;70:305–317. [PubMed] [Google Scholar]
- Jacobs K., Shoemaker C., Rudersdorf R., Neill S. D., Kaufman R. J., Mufson A., Seehra J., Jones S. S., Hewick R., Fritsch E. F. Isolation and characterization of genomic and cDNA clones of human erythropoietin. 1985 Feb 28-Mar 6Nature. 313(6005):806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Goldwasser E. Erythropoietin radioimmunoassay in evaluating patients with polycythemia. Ann Intern Med. 1981 Jan;94(1):44–47. doi: 10.7326/0003-4819-94-1-44. [DOI] [PubMed] [Google Scholar]
- Krantz S. B., Goldwasser E. Specific binding of erythropoietin to spleen cells infected with the anemia strain of Friend virus. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7574–7578. doi: 10.1073/pnas.81.23.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWY P. H., KEIGHLEY G., BORSOOK H. Inactivation of erythropoietin by neuraminidase and by mild substitution reactions. Nature. 1960 Jan 9;185:102–103. doi: 10.1038/185102a0. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Lee-Huang S. Cloning and expression of human erythropoietin cDNA in Escherichia coli. Proc Natl Acad Sci U S A. 1984 May;81(9):2708–2712. doi: 10.1073/pnas.81.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. D. The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem Soc Symp. 1974;(40):17–26. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miyake T., Kung C. K., Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977 Aug 10;252(15):5558–5564. [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. The pathway of eukaryotic mRNA formation. Annu Rev Biochem. 1983;52:441–466. doi: 10.1146/annurev.bi.52.070183.002301. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Analysis of processing and polyadenylation signals of the hepatitis B virus surface antigen gene by using simian virus 40-hepatitis B virus chimeric plasmids. Mol Cell Biol. 1983 Dec;3(12):2250–2258. doi: 10.1128/mcb.3.12.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Sam J., Simmer R. L., Keith D. H., Shively L., Teplitz M., Itakura K., Gartler S. M., Riggs A. D. Isolation of a cDNA clone for human X-linked 3-phosphoglycerate kinase by use of a mixture of synthetic oligodeoxyribonucleotides as a detection probe. Proc Natl Acad Sci U S A. 1983 Feb;80(3):802–806. doi: 10.1073/pnas.80.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlaub G., Chasin L. A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Woo S. L. A sensitive and rapid method for recombinant phage screening. Methods Enzymol. 1979;68:389–395. doi: 10.1016/0076-6879(79)68028-x. [DOI] [PubMed] [Google Scholar]
- Zanjani E. D., Ascensao J. L., McGlave P. B., Banisadre M., Ash R. C. Studies on the liver to kidney switch of erythropoietin production. J Clin Invest. 1981 Apr;67(4):1183–1188. doi: 10.1172/JCI110133. [DOI] [PMC free article] [PubMed] [Google Scholar]