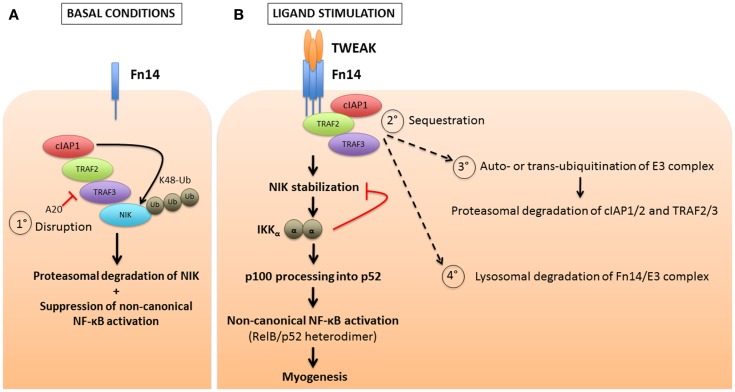
Figure 3.
cIAP1/2 regulation of TWEAK-induced non-canonical NF-κB pathway activation. (A) Contrary to the canonical NF-κB pathway for which cIAP1/2 are positive regulators, these two E3 ubiquitin ligases act, via the bridging molecules TRAF2 and TRAF3, as negative regulators of the non-canonical NF-κB by continuously degrading the NF-κB-inducing kinase, NIK. This occurs through the attachment of K48-linked polyubiquitin chains and the targeting of NIK to the proteasome, under basal or non-stimulated conditions. One mechanism (process 1°) to reverse this inhibitory effect is through A20 mediated disruption of the cIAP-TRAF complex, which would presumably lead to ligand-independent activation of the non-canonical NF-κB pathway. (B) In most instances, upon stimulation of a TNF receptor superfamily member by its ligand, the cIAPs and TRAFs are recruited away from the cytosolic reactions and sequestered at the plasma membrane (process 2°). This allows for the stabilization of NIK, the formation of IKKα homodimers, and ultimately the partial processing of p100 into p52. RelB and p52 then dimerize to form an active, functional NF-κB transcription factor complex. Several models of receptor-mediated non-canonical NF-κB activation have been proposed, which include the cIAPs inducing K48-linked ubiquitination of themselves and the TRAFs, resulting in their proteasomal degradation (process 3°). Alternatively, the receptor-mediated endocytosis of the TWEAK-Fn14 complex results in lysosomal degradation of the cIAPs and TRAFs (process 4°). This loss of cIAP and TRAF adaptors may impact other pathways, such as CD40L signaling through CD40, that also require these adaptors.