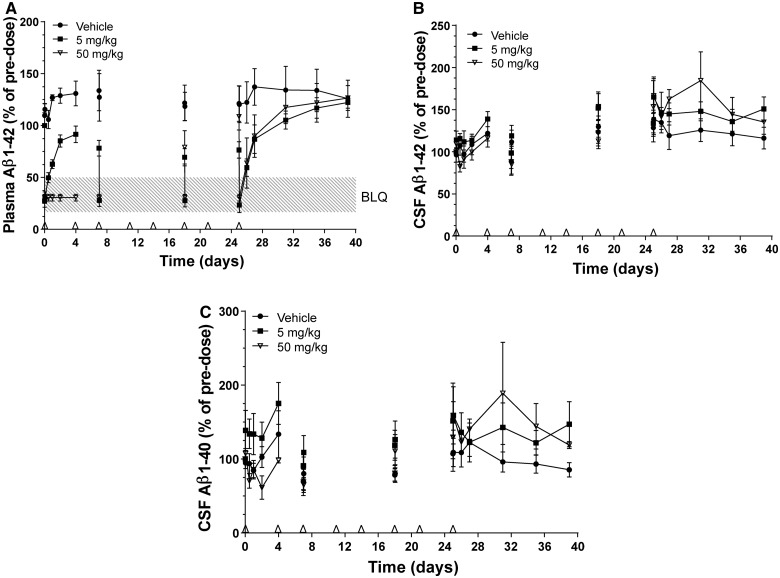
Figure 4.
Amyloid-β1–40 (Aβ1–40) and/or amyloid-β1–42 (Aβ1–42) concentrations in plasma and CSF of male cynomolgus monkeys with implanted CSF catheters after twice-weekly intravenous administration of HSA-hNEPv (0, 5 and 50 mg/kg) for 1 month. Arrows denote times of dosing. Amyloid-β levels on Days 7, 18 and 25 are shown pre- and post-dose to illustrate the degree of suppression during the study. All plots include animals where exposure was reduced presumably as a consequence of development of anti-drug antibodies. (A) Mean (± SEM) amyloid-β1–42 concentrations (expressed as per cent of pre-dose) in plasma after dosing of HSA-NEPv at 0 (filled circle), 5 (filled square) and 50 (open inverted triangle) mg/kg. Pre-dose plasma amyloid-β1–42 concentrations ranged from 20–50 pg/ml. (B) Mean (± SEM) amyloid-β1–42 concentrations in CSF after dosing HSA-NEPv at 0 (filled circle), 5 (filled square) and 50 (open inverted triangle) mg/kg. Pre-dose CSF Aβ1–42 concentrations ranged from 300–1000 pg/ml. (C) Mean (± SEM) amyloid-β1–40 concentrations in CSF after dosing at 0 (filled circle), 5 (filled square), and 50 (open inverted triangle) mg/kg. Pre-dose CSF amyloid-β1–40 concentrations ranged from 400–3500 pg/ml.