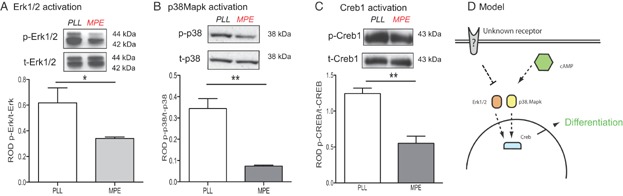
Figure 1.
Inhibition of OPC differentiation by MAI is regulated by phosphorylation of Erk1/2, p38Mapk and Creb1.
A–C. Top, representative blots of phosphorylated (p-) protein, re-probed for total (t-) protein levels, of (A) Erk1/2, (B) p38Mapk and (C) Creb1. Bottom, relative optical-density-based (ROD) quantification of phosphorylated to total protein ratios of Erk, p38Mapk and Creb1 on control (poly-l-Lysine, PLL) substrates and in the presence of MAI (MPE). Student t-tests: (A) n = 3; *p < 0.05; PLL:0.6177 ± 0.1166; MPE: 0.3406 ± 0.01203; B) n = 3; **p < 0.01; PLL: 0.3438 ± 0.04692; MPE: 0.07413 ± 0.004414; (C) n = 4; **p < 0.01; PLL: 1.244 ± 0.07505; MPE: 0.5517 ± 0.09847.
D. Proposed model of Mapk signalling during OPC differentiation: OPC differentiation is mediated by activation of Erk1/2 and p38Mapk. Erk1/2 and p38Mapk in turn activate Creb1, which ultimately leads to OPC differentiation. The presence of MAI impairs Erk1/2 and p38Mapk activation, which results in impairment of Creb1 phosphorylation. The model predicts that an increase of intracellular cAMP results in activation of p38Mapk, Erk1/2 and Creb1 activity and ultimately promotes OPC differentiation. Error bars: SEM.