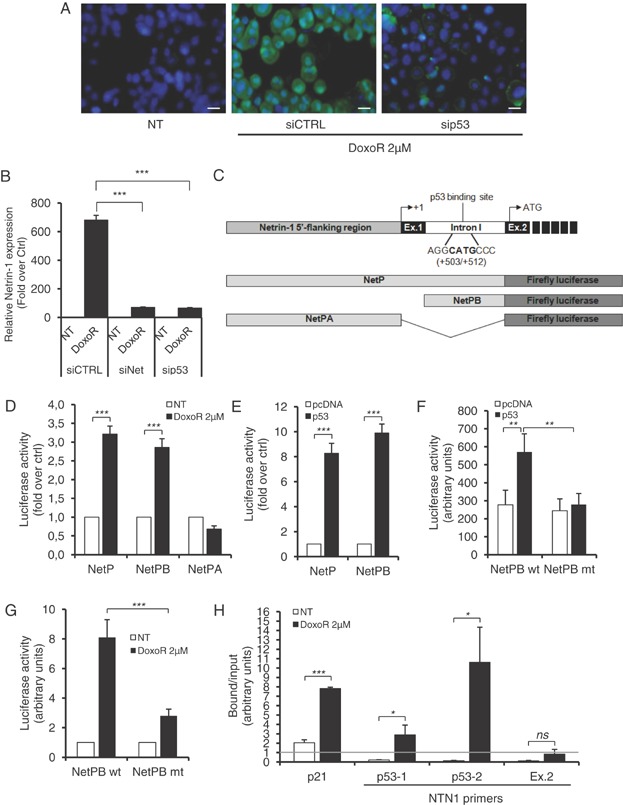
Figure 6.
Netrin-1 upregulation is p53-dependent.
A. A549R cells were transfected with p53 siRNA (sip53) or control siRNA (siCTRL) and treated with 2 µM Doxorubicin for 48 h. Endogenous netrin-1 protein (green) was detected by immunofluorescence staining. Nuclei were counterstained with Hoescht staining (in blue). While Doxorubicin strongly increased netrin-1 protein, p53 silencing was able to revert this upregulation. White scale bar: 20 µm.
B. Netrin-1 expression levels in p53- and netrin-1-silenced A549R cells, treated with 2 µM Doxorubicin for 24 h, were evaluated by quantitative RT-PCR.
C. Schematic representation of the 5′-flanking netrin-1 genomic region. The 5′-flanking region, containing the netrin-1 promoter A (grey box) (Paradisi et al, 2008), the first two exons (solid box), the first intron (open box), containing the alternative promoter B, described in(Delloye-Bourgeois et al, 2012), the transcription start site +1, and the putative p53 binding site, are indicated. The different netrin-1 promoter A (NetPA) and B (NetPB) region, and the full promoter region (NetP) were cloned upstream the firefly luciferase gene.
D. A549R cells were transfected with the indicated netrin-1 promoter constructs and treated with 2 µM Doxorubicin for 24 h. Netrin-1 promoter activity was then evaluated by measuring luciferase activity, as indicated in the materials and methods section.
E. A549R cells were co-transfected with the netrin-1 promoter constructs NetP and NetPB and p53, and luciferase activity was measured 48 h later. Over-expression of p53 was able to trans-activate both the promoter constructs.
F,G. Mutation of the putative p53 binding site abolished netrin-1 promoter trans-activation by Doxorubicin or p53 over-expression. A549R cells were transfected with NetPB promoter construct wild-type (NetPB wt) or mutated for the p53 binding site (NetPB mut). While treatment with Doxorubicin (F) or p53 over-expression (G) was able to trans-activate the wild-type netrin-1 promoter, abolishing p53 binding was sufficient to decrease netrin-1 promoter activation.
H. Chromatin Immunoprecipitation (ChIP) assay was performed in A549R cells treated with 2 µM Doxorubicin for 24 h, as described in the material and methods section. p53-co-precipitated DNA was analysed by quantitative PCR. Two different couples of primers, surrounding the p53 binding site, were used for netrin-1 (p53-1 and p53-2). A couple of primers, able to amplify a DNA fragments corresponding to netrin-1 exon 2 (Ex.2), were used as negative control. p21 primers, surrounding the p53 binding site -1354 contained in the p21 promoter(Macleod et al, 1995), were used as positive control for p53 activation and binding. While in control, not treated cells p53 was not able to bind netrin-1 genomic DNA, in Doxorubicin-treated cells we observed a strong enrichment of netrin-1 DNA surrounding the p53 binding site, but not in the netrin-1 DNA corresponding to exon 2, in the p53 co-immunoprecipitate. Grey lane represents the threshold of enriched DNA region that co-immunoprecipitate with p53. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant; NT, not treated; DoxoR, Doxorubicin.