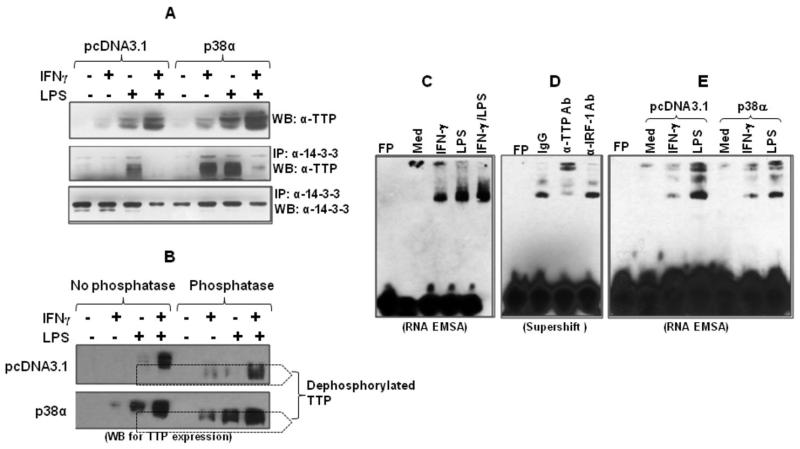
Fig. 7. Overexpression of p38 MAPK inhibits TTP binding to the p19 3′UTR.
(A) 5 μg of p38 expression or empty vectors were transiently transfected into RAW cells. 24 h later the cells were stimulated with IFN-γ (10 ng/ml), LPS (1 μg/ml), or IFN-γ plus LPS for 4 h, followed by extraction of whole cell lysates to detect TTP protein by Western blot (upper panel). 100 μg of cytoplasmic protein was immunoprecipitated with 2 μg of anti phospho-14-4-3 antibody and the IPed products were used to detect TTP expression with anti-TTP antibody (middle panel). The same blot was stripped and reanalyzed with anti-phospho-14-3-3 antibody (lower panel). (B) RAW cells were transiently transfected with p38-α or empty vector pcDNA3.1 as described above and stimulated by IFN-γ (10 ng/ml), LPS (1 μg/ml) or LPS plus IFN-γ for 2 h, followed by extraction of whole cell lysates. 50 μg of lysates were digested with 50 U lambda protein phosphates for 2 h at 30°C. The digested product and equal amount of undigested lysates were used for detection of TTP expression by western blot. (C) RNA-LightShift Chemiluminescent EMSA was performed by incubating biotin-labeled RNA probe with 10 μg cytoplasmic protein. Cytoplasmic proteins were isolated from RAW cells treated with IFN-γ (10 ng/ml), LPS (1 μg/ml) and IFN-γ plus LPS for 4 hrs as we previously described. Med: medium condition. (D) 10 μg of cytoplasmic protein was incubated with 1 ug of anti-TTP antibody and unrelated anti-IRF-1 antibody for 20 min, followed by performing RNA-EMSA assay. (E) 5 μg of p38 expression or empty vectors were transiently transfected into RAW cells. 24 h later the cells were stimulated with IFN-γ (10 ng/ml) or LPS (1 μg/ml) for 4 h, followed by extraction of cytoplasmic protein to detect TTP binding by RNA-EMSA. 10 μg of protein was used for each sample. Data shown are one of two-three experiments with similar results.