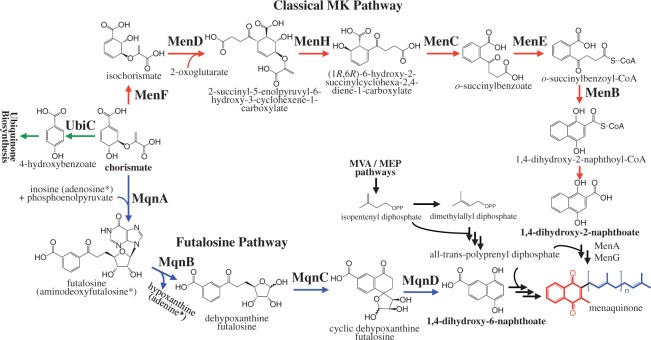
Fig. 1.—
Schematic representation of menaquinone biosynthetic pathways. MenF: isochorismate synthase, including Chorismate_bind (Pfam: PF00425) protein domain; MenD: 2-succinyl-5-enolpyruvyl-6-hydroxy-3-cyclohexene-1-carboxylate synthase, including TPP_enzyme_C (PF02775), TPP_enzyme_M (PF00205), and TPP_enzyme_N (PF02776) domains; MenH: (1R,6R)-6-hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate synthase, including Abhydrolase_1 (PF00561) domain; MenC: o-succinylbenzoate synthase, including MR_MLE (PF01188) and MR_MLE_N (PF02746) domains; MenE: o-succinylbenzoate-CoA ligase, including AMP-binding (PF00501) domain; MenB: 1,4-dihydroxy-2-naphthoyl-CoA synthase, including ECH (PF00378) domain; MenI: 1,4-dihydroxy-2-naphthoyl-CoA thioesterase, including 4HBT (PF03061) domain; MqnA: futalosine synthase, including VitK2_biosynth (PF02621) domain; MqnB: futalosine hydrolase, including PNP_UDP_1 (PF01048) domain; MqnC: dehypoxanthinyl futalosine cyclase, including Radical_SAM (PF04055) and Radical_SAM_N (PF08497) domains; MqnD: 1,4-dihydroxy-6-naphthoate synthase, including VitK2_biosynth (PF02621) domain. *Adenosine could be a precursor to synthesize aminodeoxyfutalosine via MqnA (Arakawa et al. 2011). The nonpolar moiety of menaquinone is displayed in blue, and the polar moiety is displayed in red.