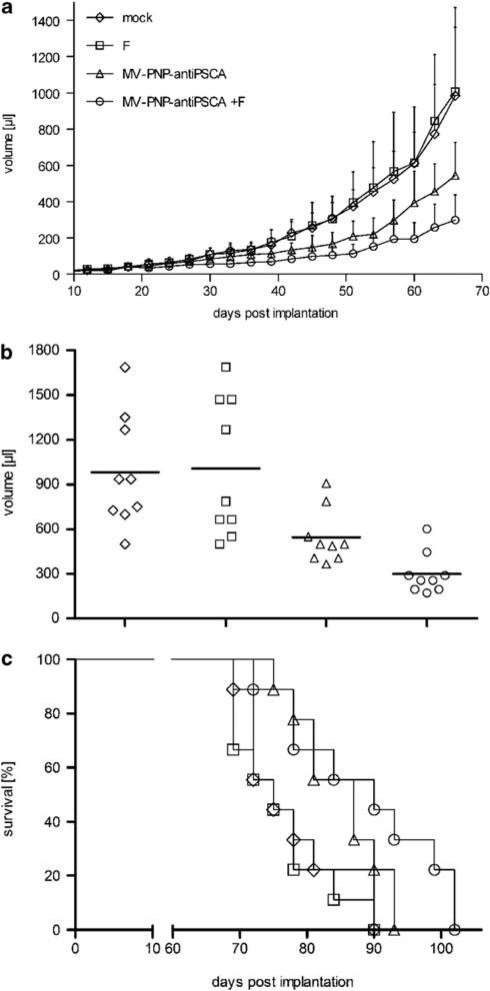
Figure 4.
Oncolytic effects of the measles virus (MV)-purine nucleoside phosphorylase (PNP)-anti-PSCA/fludarabine system in non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice after intratumoral (i.t.) administration. BxPC-3 cells (6.6 × 106) were implanted into the right flank of mice (9 per group) and treatment started at a tumor volume of ca. 50 μl. Mice were injected i.t. on 5 consecutive days (days 19–23) with a dose of 7.2 × 105 cell infectious unit (ciu) of MV-PNP-anti-PSCA each. Fludarabine was given intraperitoneally (i.p.) (250 mg/kg per dose) 3 days after the last MV application on 3 consecutive days (diamond, mock-treated; square, treated with fludarabine only; triangle, MV-PNP-anti-PSCA only; circle, MV-PNP-anti-PSCA + fludarabine). (a) Tumor volume measurements starting on day 3 after subcutaneous implantation (error bars are indicating standard deviation of the mean). (b) Distribution of tumor volumes at day 66 after implantation. Each dot represents one mouse with 9 mice per group. (c) Kaplan–Meier survival curve documenting the effects of oncolytic MV-PNP-anti-PSCA and prodrug, respectively. The defined end point was >1500 μl of tumor volume.