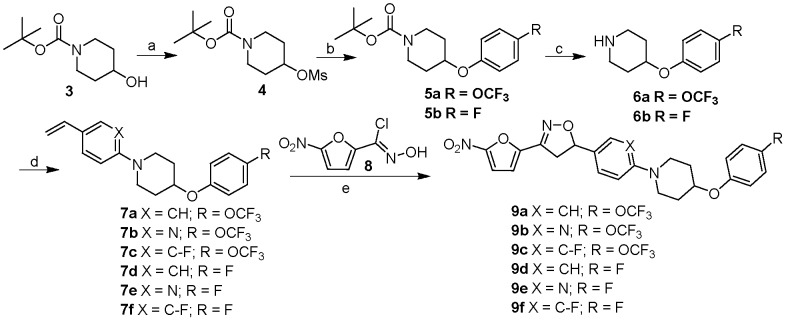
Figure 2. Synthesis of pentacyclic nitrofurans.
Reagents and conditions: a) MsCl, Et3N, CH2Cl2, RT, 2 h, 94%; b) substituted phenol, nBu4NCl, H2O, 100°C, 12 h, 86–90%; c) TFA, CH2Cl2, RT, 1 h, 92–95%; d) aryl bromide, 2-(di-tert-butylphosphino)biphenyl, NaOtBu, Pd(OAc)2, toluene, 100°C, 3 h, 50–70%; e) 8, Et3N, CHCl3, RT, 3 h, 50–68%.