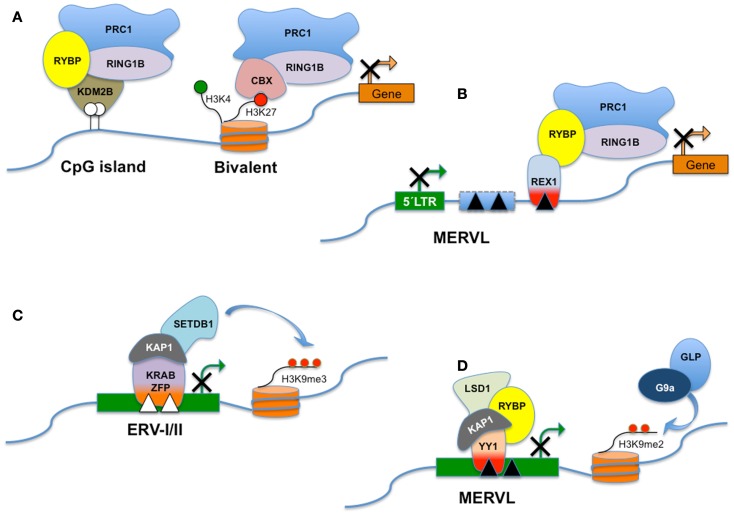
Figure 3.
RYBP and distinct PRC1-like complexes. Polycomb Group genes (PcG) assemble into multiprotein complexes and, depending on the subunit composition, the complexes are recruited to different types of target sites. PRC1 (Polycomb repressive complex 1) is a canonical complex composed of either RING1A/or RING1B, MPH1/2/3, CBX2/7/8, and one of the Polycomb group RING fingers proteins (PCGF2–5) and is referred to here as CBX-PRC1 (A). CBX-PRC1 mediates H3K27me3-dependent silencing of bivalent promoters. PcG-regulated promoters devoid of H3K27me3 recruit a RYBP-containing variant complex termed RYBP-PRC1, which appears similar to a KDM2B-containing complex that recognizes non-methylated CpG islands (A). For clarity, only the subunits that differ between CBX-PRC1 and RYBP-PRC1 are shown, in addition to RING1B. RYBP silences the expression of transposable elements, including MERVL. RYBP can interact with both REX1 and/or RING1B. In this hypothetical model, REX1 might tether RYBP and the polycomb silencing complex to specific binding sites in MERVL or MERVL-derived control elements (B). Repression of class I and class II ERVs (C) is dependent on SETDB1 histone H3 lysine 9 trimethylase activity (H3K9me3). SETDB1 is believed to be recruited to its ERV targets via KRAB-containing zinc finger proteins and their co-repressor KAP1. MERVL repression is dependent on the presence of H3K9Me2 deposited through the combined action of G9a/GLP (D). Surprisingly, deletion of a variety of other chromatin proteins including LSD1, RYBP, and KAP1 also derepresses MERVL. The interplay between these pathways is presently unknown. LSD1/KAP1 and RYBP might all be recruited trough YY1-family DNA-binding factors as YY1 independently interacts with either KAP1 or RYBP (D).