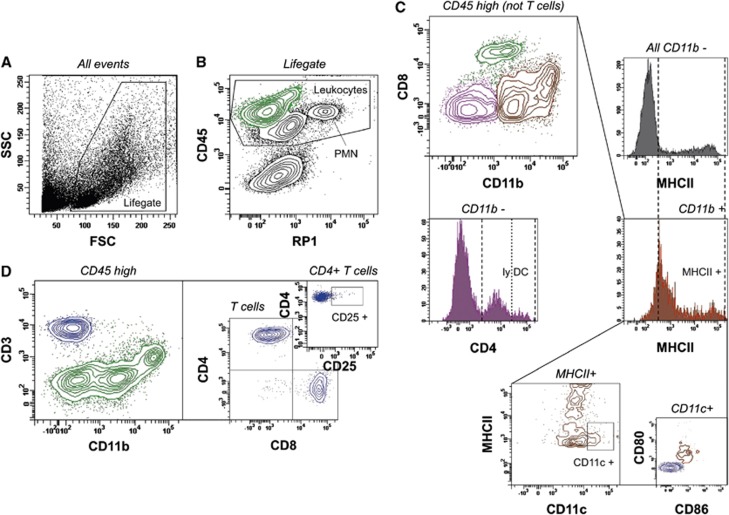
Figure 1.
Gating strategy for the analysis of resident and brain-infiltrating leukocytes. Initially, viable leukocytes were detected based on morphologic parameters (A) and CD45 expression (B). CD45 intermediate (int) neutrophils were characterized by RP1 expression (B, PMN), and by being CD45 int/CD11b+/CD80−/CD86−/MHCII−. Microglia was identified by a CD45 low/RP1− profile (B) and further denoted as CD11b+/CD3−/CD8−/CD4±cells. Total CD45 high cells (B, green) were further discriminated (C, D). CD3−/CD11b int/CD8+ events were classified as natural killer cells (C, green, for additional confirmation of their identity refer to Supplementary Figure 1). CD3−/CD11b−/CD8− cells (C, purple) could be divided into a CD4− and a CD4+ type (C, purple histogram), at which the CD4+ subtype was considered as lymphoid dendritic cells (lyDC). CD11b+ cells (C, ocher) were classified as myeloid cells composed of monocytes, macrophages, and dendritic cells (DC). These cells were further discriminated into MHCII− monocytes and MHCII+ macrophages/DC (ocher histogram, plus depiction of total CD11b− cells in the gray histogram). The latter population could be further distinguished into MHCII int/CD11c+/CD80+/CD86+ myeloid DC (C, medium and small plot, including comparative display of T lymphocytes). T cells were identified being CD3+/CD11b− (D, blue) and subsequently discriminated into CD8+ cytotoxic T cells, CD4+ T helper cells and few CD8−/CD4− cells (D, medium plot). A further small T-cell subpopulation was classified by CD4 and CD25 expression (D, small plot). FSC, forward light scatter; SSC, side light scatter.