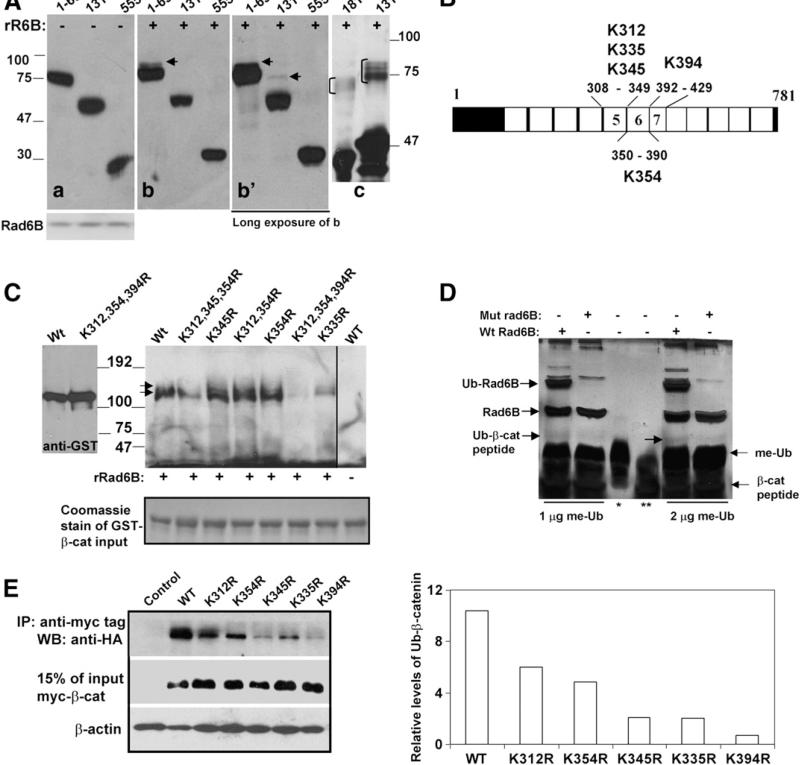
Fig. 3.
Lysine 394 of β-catenin is a major Rad6B ubiquitination site. (A) Cytosols from COS7 cells transfected with indicated myc-tagged β-catenin (a, b, b′) or purified GST-β catenin (181–422 and 131–422; panel c) were subjected to in vitro ubiquitination assays with recombinant Rad6B (panels b, b′, and c). Panel a, control reactions performed without Rad6B. Panel b′ is a longer exposure of b. Anti-myc and GST-tag reactive ubiquitinated β-catenin are indicated by arrows in b and b′, and bracket in c, respectively. (B) Schematic representation of lysines in ARMs 5–7. (C) Rad6B ubiquitination of purified GST-wild type (Wt) or K to R substituted β-catenin proteins. Last lane, wild type β-catenin reaction lacking Rad6B. Immunoblots were probed with ubiquitin antibody. Arrows indicate the positions of the ubiquitinated doublet bands of β-catenin. Left blot, GST-β-catenin proteins without reaction and detected by GST tag antibody. Bottom panel, Coomassie staining of GST-β-catenin proteins used in the reactions. (D) In vitro ubiquitination of K394 containing β-catenin peptide performed in the presence of wild type (Wt) or catalytically inert (mut) Rad6B and methylated ubiquitin (me-Ub). Lanes *, ** indicate me-Ub and β-catenin peptide, respectively. Reaction products were detected by silver staining. (E) In vivo ubiquitination of myc-tagged wild type or mutant β-catenin in MCF-7 cells. Right panel, graphic representation of wild type and mutant β-catenin ubiquitination.