Abstract
Regionalization of health care is a method to provide high quality, cost-efficient health care to the largest number of patients. Within pediatric medicine, regionalization has been undertaken in two areas: neonatal intensive care and pediatric trauma care. The supporting literature for the regionalization of these areas demonstrates the range of studies within this field: studies of neonatal intensive care primarily compare different levels of hospitals, while studies of pediatric trauma care primarily compare the impact of institutionalizing a trauma system in a single geographic region. However, neither specialty has been completely regionalized, possibly because of methodologic deficiencies in the evidence base. Research with improved study designs, controlling for differences in illness severity between different hospitals; a systems approach to regionalization studies; and measurement of parental preferences will improve the understanding of the advantages and disadvantages of regionalizing pediatric medicine and ultimately optimize the outcomes of children.
Keywords: Neonatal Intensive Care, Pediatric Trauma Care, Regionalization of Care, Health Care Systems
The phrase “regionalization of health care” suggests the development of a structured system of care “to improve patient outcomes by directing patients to facilities with optimal capabilities for a given type of illness or injury.”1 The development of a regionalized system is typically driven by economic factors, such as the infeasibility of all hospitals to maintain the equipment and personnel to treat specific medical conditions,2 or by interhospital variations in patient outcomes within a geographic region.
The development of these regionalized systems is not the same as the development of regional centers of care. Competitive market forces focusing on improved outcomes create regional centers of excellence where patients choose to receive care. Examples of such regional centers include adult cancer care and complex surgical procedures. However, a number of conditions, such as adult cardiac care and stroke management,3-6 are less amenable to these free market principles because their acute presentation does not allow patients to make informed choices on where they will receive care. For these conditions, regionalized systems ensure that patients receive the early care necessary to optimize their outcomes.7
Unfortunately, the evidence base for regionalized systems in pediatric medicine has lagged behind the development of such systems. The purpose of this article is to (1) present a conceptual framework for the development of regionalized health care systems; (2) describe types of evidence for regionalized systems; (3) describe the overall deficiencies in the current literature; and (4) outline a plan for future research. This review will use neonatal intensive care8 and pediatric trauma care9 as examples for other pediatric conditions that, in the future, may be amenable to a more regionalized approach to care. In both areas, hospitals are already given a “level” of care based on their capabilities that differ between these two conditions (Table 1).9, 10
Table 1.
Levels of Care for Neonatal Intensive Care Units and Pediatric Trauma Centers
| Levels of Pediatric Trauma Care9 | |
|---|---|
| Level | Definition |
| I | Regional resource trauma center |
| II | Trauma center, may not be able to provide comprehensive care for the most severely injured patients |
| III | Hospital provides assessment, resuscitation, emergency operations, and stabilization of patients prior to transfer to facility that provides definitive trauma care |
| IV | Facility provides advanced trauma life support prior to patient transfer |
| Levels of Neonatal Intensive Care Units10 | |
| Level | Definition |
| I | Basic neonatal care available |
| II | Specialty neonatal care available, primarily ≥ 1500 grams at birth |
| III | Subspecialty neonatal care available |
| IIIA | Hospital or state-mandated restriction on type and/or duration of mechanical ventilation |
| IIIB | No restriction on mechanical ventilation; no major surgery |
| IIIC | All surgeries except congenital heart disease repair and extra-corporeal membrane oxygenation |
| IIID | All surgeries |
Conceptual Framework
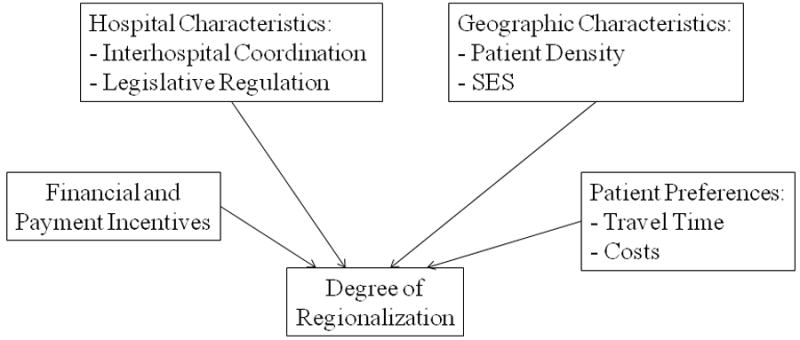
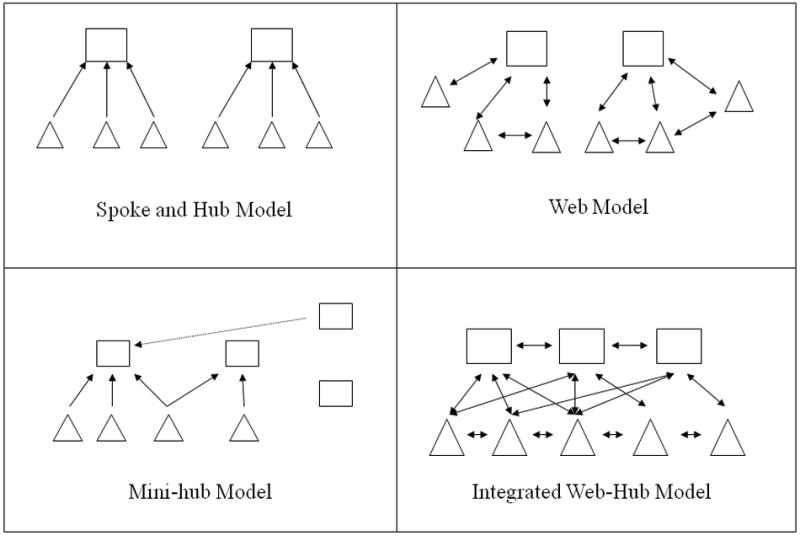
Regionalization improves patient outcomes through two primary mechanisms (Table 2): improved outcomes at high volume, high specialty centers; and improved coordination of care within a given geographic area. Regionalization may take several forms (Figure 1). Models such as the spoke-and-hub system and the web system are characterized by a specialized center that manages the most complex patients within a geographic area, supported by less specialized hospitals. The degree of coordination between hospitals helps distinguish the two systems. The “mini-hub” model is a less coordinated, “de-regionalized” system that may develop depending on financial incentives, hospital and geographic characteristics, patient preferences, and lack of coordination between centers (Figure 2).
Table 2.
Mechanisms through which regionalization of care improves outcomes
| Mechanism | Advantages | Disadvantages |
|---|---|---|
|
| ||
| 1. High-acuity patients receive care at facilities with necessary resources | Resources at medical centers where patients receive care match needs of patients | Diminished volume-outcome relationship if increased numbers of specialty hospitals are built |
| Increased fixed costs and resources to develop such capability | ||
| Longer travel time and costs to patients and families | ||
| Economic inefficiency if supply does not meet demand | ||
|
| ||
| 2. Improved systems of care | ||
|
| ||
| 2a. Increased coordination of care and education | Better coordination of care during the acute phase of hospitalization | Requires cooperation between multiple centers beyond transferral of patients |
| Improved standardization of care within network of non-specialty hospitals | Typically requires state or regulatory intervention into health care system | |
| Poor coordination of post-discharge follow-up care. | ||
|
| ||
| 2b. Off-site consultation and assistance in the management of patients | Improved provision of scarce services to non-specialty hospitals | Limited number of conditions amenable to method |
| Allow patients to remain at nearby hospitals, reducing travel time and costs | Limited evidence base | |
| Requires cooperation and collaboration between specialty and non-specialty hospital | ||
Figure 1.
Various forms of regionalization systems. Squares represent central, specialty hospitals, while the triangles represent non-specialty hospitals. Arrows represent the direction of patients within the system. The spoke-and-hub system and the web system are characterized by a specialty center that manages patients referred by outlying non-specialty hospitals. The degree of coordination between the two types of hospitals distinguishes between these two systems. The mini-hub model is characterized by an expansion of specialty hospitals that have differing degrees of interaction with the transport system, non-specialty hospitals, and with other specialty hospitals. The integrated web approach is characterized by seamless communication and coordination between specialty and non-specialty hospitals.
Figure 2.
Conceptual framework for the development of regional systems. Various factors contribute to the development of both the type of regionalized system described in Figure 1 and the overall degree of regionalization.
Types of Regionalization Studies and their Outcomes
1) Studies of individual hospitals: Improved outcomes at specialty hospitals
One recognized outcome of regionalization research is that the management of complex conditions at specialized hospitals may improve outcomes. For example, studies from California found that premature infants delivered at lower level or lower volume neonatal intensive care units (NICUs) had a 19-272% increased odds of mortality.11-13 Older studies14-16 and other recent studies from Colorado, South Carolina, and Illinois17-19 show similar results.
In contrast, although evidence suggests that injured adult patients have improved outcomes at definitive trauma centers,20, 21 state-specific or single institution studies have not consistently shown improved outcomes for pediatric trauma victims treated at pediatric trauma centers or high volume centers.22-27 One national study demonstrated that pediatric trauma victims have improved outcomes when cared for at free-standing children’s hospitals.28 However, this comparison disregards confounding from trauma level. The current classification of pediatric trauma centers lacks uniformity, making large-scale evaluations of outcomes by trauma level extremely difficult. Although performing single center or single state studies standardize this classification, these studies are poorly generalizable to the national trauma system. Thus, in 2007, a scientific review concluded that there was insufficient evidence to determine where pediatric trauma victims have the best outcomes.29
2) Studies of geographic systems: Increased geographic coordination of care and knowledge transfer
Regionalization of care may also improve patient outcomes through increased coordination of care and knowledge transfer between central, specialized hospitals and non-specialty hospitals. Measured from a population perspective, implementation of a trauma system provides a 15 to 20% improvement in survival rate among seriously injured patients.30 The effect of regionalized pediatric trauma care shows similar improvement in mortality for injury and motor vehicle patients. 31, 32
Unlike pediatric trauma care, there are few studies of the impact of neonatal intensive care systems on perinatal health and outcomes. Many studies occur outside the United States. 33, 34 The one randomized study performed in the late 1970s suggests that a regionalized system increases deliveries of very-low-birth weight infants at specialty hospitals without significantly lower mortality.8 More recent studies have examined the impact of perinatal “de-regionalization” within a single geographic area.18, 35-39 There have been few attempts to measure the impact of these poorly organized de-regionalization programs on outcomes within a geographic area. As with pediatric trauma studies, single state studies have limited generalizability and statistical power to detect a difference in outcomes.
Moderating factors for the development of regionalized systems
There are a number of factors that help determine both the degree of regionalization and the type of system that develops in any given geographic area (Figure 2). Although there are no studies of the relationship between geographic factors such as population density, the composition of the physician workforce, and the type of system that develops, studies of other modulating factors help explain the development of various regionalized systems of care.
Financial Incentives
Financial incentives play a role in the development of different types of regionalized systems. The regional model of neonatal intensive care began to weaken in many areas of the United States by the 1990s.35, 36, 40 For example, in California between 1990 and 1997, the percentage of very low birth weight births at regional perinatal centers declined from 36.5% to 27.2%, and the percentage of these births at community hospitals increased from 11.7% to 37.4%.39 The economic incentives in obstetric care help explain some of this expansion of specialty NICUs. Fees for the delivery of the infant are much higher than the fees, if any, given for the provision of prenatal care. Thus, many hospitals are under economic pressure to open a specialty NICU to reduce the transfer of high-risk women to a central, specialty unit. These community specialty NICUs typically do not have the capability to manage all premature infants delivered at their hospital, resulting in a “mini-web” model of health care, where there are multiple large and small volume specialty centers that vary widely in their transport capability and coordination of care with non-specialty and other specialty units (Figure 2). Although these community specialty hospitals may meet the evidence provided by studies that focus on the improved outcomes of patients treated at specialty hospitals, such systems undermine the original intent of regionalized care by limiting the number of infants transferred to central, high volume hospitals. These factors result in maldistributed capacity. A comprehensive examination of the outcomes and costs of such systems is essential to optimizing the outcomes of premature infants.
In pediatric trauma, the financial incentives of care are quite different. Many hospitals do not profit from the provision of pediatric trauma care. Thus, an estimated 28.5% of the pediatric population lacks prompt access to high-level pediatric trauma care, while other regions have multiple hospitals with high-level pediatric trauma capabilities.41 As with neonatal intensive care, the expansion of pediatric trauma care has not historically matched the needs of the population. Remedies to this mismatch may require expansion of high-level pediatric trauma centers into new regions or the development of a coordinated care approach.
Coordination of Care between Hospitals
Recently there has been interest in using alternatives to patient transport for the coordination of patient care. One feature that underlies the more interrelated “web” models of regionalized care is the amount of coordination between various specialty hospitals and between specialty and non-specialty hospitals. The most prominent alternative has been the use of technology such as telemedicine to deliver the doctor, albeit virtually, to the patient. For example, telemedicine may allow a patient to be kept at a local, less specialized center when access to a subspecialist is the primary reason to transfer a patient. This practice is very amenable to disciplines that rely on pattern recognition and cognitive decision making, including radiologists interpreting radiographs,42 ophthalmologists examining the retina of a premature infant,43, 44 dermatologists examining rashes, and vascular neurologists examining patients and reviewing imaging studies.4, 6, 45 Even for conditions that may require procedural interventions, such as critical care medicine, telemedical consultation has been used to assist in the management of patients prior to transfer.46 The effectiveness of such remote interactions, however, is uncertain, because of small sample sizes47 or technological barriers to accurate image quality.48
An additional challenge to these telemedicine models is the continued need for a baseline level of specialist care or cognitive knowledge at the non-specialty unit. These services can extend beyond physicians, including the quality of nursing staff and specialized equipment that may be available only at the specialty hospital. Rigorous observational or experimental studies of the costs and benefits of these treatment modalities are still needed.
Patient preferences for treatment location
Patient preferences for treatment location are poorly understood. Adult patients tend to receive obstetric and medical care at the nearest medical center,49, 50 and regionalization has increased travel times for adults undergoing surgical procedures or angioplasty for myocardial infarction.51, 52 The role of travel time in pediatric medicine is unknown. Also, regionalized systems rely on the back-transport, or repatriation, of patients to non-specialty hospitals once the acuity of the patient improves. Without such a system, the specialty hospitals could be overcrowded with patients whose care is better matched to other hospitals. Recent data from 236 neonatal families at two East Coast hospitals found that less than 50% of families desired back-transport, because of their comfort with the caregiver team at the high-level hospital.53 Consistent communication between the provider and family is a critical element to a smooth back-transport.54 Most studies of back-transport suffer from the biased selection of patients chosen for back-transport, compared to those who remained at the specialty center until discharge.55 More research is needed on how families choose a hospital and on methods to optimize transfer of patients.
Deficiencies in the Literature
There are common deficiencies in many areas of regionalization research. These deficiencies include inadequate adjustments for casemix differences between specialty and non-specialty hospitals; a limited number of assessed outcomes; and failure to account for the quality of individual hospitals in the analysis.
Differences in casemix
Because of the observational nature of most regionalization research, the research design must account for the fact that specialty hospitals typically care for sicker patients than non-specialty hospitals. These differences can be measured using either basic markers of illness risk such as gestational age or mode of delivery in studies of premature infants, or illness severity scores, such as the Score of Neonatal Acute Physiology (SNAP) in neonatology56 and the Injury Severity Score (ISS)57 and the Trauma and Injury Severity Score (TRISS)58-60 in pediatric trauma. However, less than 30% of the articles on perinatal regionalization control for illness severity.15, 35, 61, 62 Also, these scores may be inaccurate,63-68 may rely on data unavailable in large-scale population studies, or “control” away for poor quality care that occurs when data are being collected. This latter point is potentially important for scores such as SNAP, which uses information from the first 12-24 hours after delivery to calculate the score, which could adjust for poor quality resuscitation.
Even with methods to control for casemix, there still may be unmeasured differences in the patients receiving care at specialty hospitals. In pediatric trauma, a well discriminating injury scoring system cannot control for the unmeasured factors that led an emergency medical service team to transport a pediatric trauma patient directly to a pediatric trauma center. These factors include weather, perceived severity of the injury, and the clinical stability of the patient.69 Unmeasured factors in neonatal studies include fetal heart tracing results and the severity of antepartum comorbidities such as hypertension. Earlier studies of perinatal regionalization noted that only those infants who “may survive” were transferred to a specialty hospital, and were consequently less sick. Currently, though, physicians are more likely to transfer the sicker mother even though the number of transports have decreased.39, 70 These issues demonstrate the need for other study designs to adjust for this bias in observational studies, such as propensity scores71-73 or instrumental variables approaches.74 Propensity score approaches improve the equality of the stratum of case and control patients by using risk factors to predict the likelihood of receiving a treatment, whereas an instrumental variables approach helps control for unmeasured differences in casemix by “pseudo-randomizes” patients to their delivery hospital using factors that are associated with where a patient delivers but are not directly associated with outcomes. Both strategies help control for the selection bias inherent in all regionalization studies.
Limited assessed outcomes
Mortality is the most common outcome in studies of injury and neonatal intensive care. Mortality rates are useful because mortality is clearly defined, mortality rates vary after adjusting for differences in casemix,75, 76 and there are associations between lower risk-adjusted mortality rates and some hospital-level processes of care.11 However, pediatric mortality is usually a rare event except in specific subpopulations such as very-low birth weight infants.77
Few studies examine other outcomes. Only two studies of regionalized neonatal intensive care examine neurodevelopmental outcome, which found no differences between regionalized and non-regionalized areas,78, 79 whereas one study found improved functional outcomes for severely injured children treated at pediatric trauma centers.80 Only one study examines a complication of either premature birth or injury.81 In general, differences in processes of care have also been poorly studied. One condition that has been examined is the management of splenic injury, where centers with pediatric surgeons have lower operative rates than centers with adult surgeons.82-86 Differences in other processes of care between regionalized and de-regionalized areas, either within pediatric trauma care or neonatal intensive care, have not been studied.
Assessing the quality of individual hospitals within larger groupings of care level
No pediatric regionalization study accounts for differences in care delivered at individual hospitals. One study of very-low-birth weight infants estimated that measured hospital characteristics such as hospital volume predicted 9% of the variation in mortality rates between NICUs, whereas unmeasured characteristics – likely variations in quality – predicted 84% of these variations.87, 88 A similar study of 47 level I adult trauma centers found wide variations in mortality rates, especially for the most severely injured.89 There has been no such analysis between high level pediatric trauma centers. Proper methods should be used to distinguish variation related to the individual hospital and variation related to other characteristics of the hospital such as volume and level of care.
Future Areas of Research
This overview has shown that the evidence base for regionalized care, while compelling, is incomplete. Recommendations for future areas of research should focus in five areas.
Improved Study Designs
Studies should include additional outcomes of interest in addition to mortality. These outcomes may include clinical outcomes, such as functional ability or neurodevelopmental outcome; complications of care; measures of patient safety, such as infection rates or medication errors; and costs of care using cost-effectiveness or cost-utility studies.
More research should develop and validate measures of illness severity, particularly using data available in large-scale population data.
Regionalization studies should use methods to control for unmeasured differences in casemix between groups of hospitals such as instrumental variables approaches.
Studies should examine inter-hospital variations in outcomes, particularly within hospitals with similar consistently-applied characteristics such as “high level”, “high volume”, and “teaching hospitals”.
Studies should report the variables included in the risk-adjustment model and a measurement of the model’s discrimination, such as a c-statistic or area under a receiver operator curve.
Examine the impact of the implementation of regionalization in countries or smaller geographic areas.33, 34 Comparing the change in outcomes in these areas to the change in outcomes to similar areas that did not experience a change allows for valid assessments of the impact of these policy changes.
Financial Incentives
Further research should assess the role of obstetric and neonatal financial incentives, both how payment is divided between providers of prenatal versus delivery care and how alternative forms of payment, such as pay-for-performance,90 impact the degree of regionalization and their outcomes.
Developing new systems of regionalized care: Hospitals and Competition
Studies should examine new systems of regionalized care that account for both new technology and the economics of a given medical system. For example, to account for geographic areas with multiple central specialized hospitals, an “integrated web approach” may allow for a more collaborative approach to the management of both routine and complex patients (Figure 1). Studies of these models should investigate the effect of reduced hospital competition on patient outcomes and methods to encourage collaboration between specialty centers.
Measuring patient preferences
Qualitative and quantitative research studies are needed into parental preferences for treatment location during both the initial and chronic phases of treatment. These preferences can be used as outcome measures for both standard studies of regionalized care and in cost-effectiveness or cost-utility studies.
Geographic Influences on Regionalization
There are no studies of how geography, demography, and overall socioeconomic status influence the type of system that is implemented. These macro-level studies are important to understanding how regionalized systems develop and evolve over time.
Conclusion
To assess the optimal organization of pediatric health care, multiple outcomes from the perspectives of the individual, hospital, and population must be explored. Reliance on studies of one type may have unintended consequences, as seen in many systems of neonatal intensive care in the United States. Solid evidence for both the improvement in patient care and outcomes at specialty centers and the added benefit of various regional system models are needed to optimize the care of populations of patients with specialized, high-risk conditions.
Acknowledgments
This work is supported by 1-R01-HS015696 from the Agency for Healthcare Research and Quality (SA Lorch), K08HS017960 from the Agency for Healthcare Research & Quality (BG Carr) and R03HD061523 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (BG Carr).
Abbreviations
- NICUs
neonatal intensive care units
Footnotes
Financial disclosure and conflict of interest: The authors have no financial or other conflicts of interest to disclose.
References
- 1.Institute of Medicine. Committee on the Future of Emergency Care in the United States Health System, Board on Health Care Services. Washington, DC: National Academies Press; 2007. Emergency Medical Services at the Crossroads. [Google Scholar]
- 2.Menke TJ, Wray NP. When does regionalization of expensive medical care save money? Health Serv Manage Res. 2001;14(2):116–124. doi: 10.1258/0951484011912618. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Regionalizing Emergency Care: Workshop Summary. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 4.Meyer BC, Raman R, Hemmen T, Obler R, Zivin JA, Rao R, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol. 2008;7(9):787–795. doi: 10.1016/S1474-4422(08)70171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duchesne JC, Kyle A, Simmons J, Islam S, Schmieg RE, Jr, Olivier J, et al. Impact of telemedicine upon rural trauma care. J Trauma. 2008;64(1):92–97. doi: 10.1097/TA.0b013e31815dd4c4. discussion 97-98. [DOI] [PubMed] [Google Scholar]
- 6.Schwamm LH, Audebert HJ, Amarenco P, Chumbler NR, Frankel MR, George MG, et al. Recommendations for the implementation of telemedicine within stroke systems of care: A policy statement from the American Heart Association. Stroke. 2009;40(7):2635–2660. doi: 10.1161/STROKEAHA.109.192361. [DOI] [PubMed] [Google Scholar]
- 7.Carr BG, Conway PH, Meisel ZF, Steiner CA, Clancy C. Defining the emergency care sensitive condition: A health policy research agenda in emergency medicine. Ann Emerg Med. doi: 10.1016/j.annemergmed.2009.12.013. in press. [DOI] [PubMed] [Google Scholar]
- 8.McCormick MC, Shapiro S, Starfield BH. The regionalization of perinatal services. Summary of the evaluation of a national demonstration program. JAMA. 1985;253(6):799–804. [PubMed] [Google Scholar]
- 9.ACS-COT. Resources for Optimal Care of the Injured Patient. Chicago, IL: American College of Surgeons; 2006. [Google Scholar]
- 10.American Academy of Pediatrics Committee on Fetus and Newborn. Levels of neonatal care. Pediatrics. 2004;114(5):1341–1347. doi: 10.1542/peds.2004-1697. [DOI] [PubMed] [Google Scholar]
- 11.Phibbs CS, Bronstein JM, Buxton E, Phibbs RH. The effects of patient volume and level of care at the hospital of birth on neonatal mortality. JAMA. 1996;276(13):1054–1059. [PubMed] [Google Scholar]
- 12.Phibbs CS, Baker LC, Caughey AB, Danielsen B, Schmitt SK, Phibbs RH. Level and volume of neonatal intensive care and mortality in very-low-birth-weight infants. N Engl J Med. 2007;356(21):2165–2175. doi: 10.1056/NEJMsa065029. [DOI] [PubMed] [Google Scholar]
- 13.Cifuentes J, Bronstein J, Phibbs CS, Phibbs RH, Schmitt SK, Carlo WA. Mortality in low birth weight infants according to level of neonatal care at hospital of birth. Pediatrics. 2002;109(5):745–751. doi: 10.1542/peds.109.5.745. [DOI] [PubMed] [Google Scholar]
- 14.Paneth N, Kiely JL, Wallenstein S, Susser M. The choice of place of delivery. Effect of hospital level on mortality in all singleton births in New York City. Am J Dis Child. 1987;141(1):60–64. doi: 10.1001/archpedi.1987.04460010060024. [DOI] [PubMed] [Google Scholar]
- 15.Berg CJ, Druschel CM, McCarthy BJ, LaVoie M, Floyd RL. Neonatal mortality in normal birth weight babies: Does the level of hospital care make a difference? American Journal of Obstetrics and Gynecology. 1989;161(1):86–91. doi: 10.1016/0002-9378(89)90239-1. [DOI] [PubMed] [Google Scholar]
- 16.Gortmaker S, Sobol A, Clark C, Walker DK, Geronimus A. The survival of very low-birth weight infants by level of hospital of birth: A population study of perinatal systems in four states. American Journal of Obstetrics and Gynecology. 1985;152(5):517–524. doi: 10.1016/0002-9378(85)90618-0. [DOI] [PubMed] [Google Scholar]
- 17.Kamath BD, Box TL, Simpson M, Hernandez JA. Infants born at the threshold of viability in relation to neonatal mortality: Colorado, 1991 to 2003. J Perinatol. 2008;28(5):354–360. doi: 10.1038/sj.jp.7211918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menard MK, Liu Q, Holgren EA, Sappenfield WM. Neonatal mortality for very low birth weight deliveries in South Carolina by level of hospital perinatal service. American Journal of Obstetrics and Gynecology. 1998;179(2):374–381. doi: 10.1016/s0002-9378(98)70367-9. [DOI] [PubMed] [Google Scholar]
- 19.Dooley SL, Freels SA, Turnock BJ. Quality assessment of perinatal regionalization by multivariate analysis: Illinois, 1991-1993. Obstet Gynecol. 1997;89(2):193–198. doi: 10.1016/S0029-7844(96)00450-4. [DOI] [PubMed] [Google Scholar]
- 20.MacKenzie EJ, Rivara FP, Jurkovich GJ, Nathens AB, Frey KP, Egleston BL, et al. A national evaluation of the effect of trauma-center care on mortality. N Engl J Med. 2006;354(4):366–378. doi: 10.1056/NEJMsa052049. [DOI] [PubMed] [Google Scholar]
- 21.Demetriades D, Martin M, Salim A, Rhee P, Brown C, Chan L. The effect of trauma center designation and trauma volume on outcome in specific severe injuries. Ann Surg. 2005;242(4):512–517. doi: 10.1097/01.sla.0000184169.73614.09. discussion 517-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potoka DA, Schall LC, Gardner MJ, Stafford PW, Peitzman AB, Ford HR. Impact of pediatric trauma centers on mortality in a statewide system. J Trauma. 2000;49(2):237–245. doi: 10.1097/00005373-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Sherman HF, Landry VL, Jones LM. Should Level I trauma centers be rated NC-17? J Trauma. 2001;50(5):784–791. doi: 10.1097/00005373-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Nakayma DK, Copes WS, Sacco W. Differences in trauma care among pediatric and nonpediatric trauma centers. J Pediatr Surg. 1992;27(4):427–431. doi: 10.1016/0022-3468(92)90328-5. [DOI] [PubMed] [Google Scholar]
- 25.Colombani PM, Buck JR, Dudgeon DL, Miller D, Haller JA., Jr One-year experience in a regional pediatric trauma center. J Pediatr Surg. 1985;20(1):8–13. doi: 10.1016/s0022-3468(85)80382-1. [DOI] [PubMed] [Google Scholar]
- 26.Aaland MO, Hlaing T. Pediatric trauma deaths: A three-part analysis from a nonacademic trauma center. Am Surg. 2006;72(3):249–259. [PubMed] [Google Scholar]
- 27.Serleth HJ, Cogbill TH, Perri C, Lambert PJ, Ross AJ, 3rd, Thompson JE. Pediatric trauma management in a rural Wisconsin trauma center. Pediatr Emerg Care. 1999;15(6):393–398. doi: 10.1097/00006565-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Densmore JC, Lim HJ, Oldham KT, Guice KS. Outcomes and delivery of care in pediatric injury. J Pediatr Surg. 2006;41(1):92–98. doi: 10.1016/j.jpedsurg.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Ochoa C, Chokshi N, Upperman JS, Jurkovich GJ, Ford HR. Prior studies comparing outcomes from trauma care at children’s hospitals versus adult hospitals. J Trauma. 2007;63(6 Suppl):S87–91. doi: 10.1097/TA.0b013e31815acc0f. [DOI] [PubMed] [Google Scholar]
- 30.Mullins RJ, Mann NC. Population-based research assessing the effectiveness of trauma systems. J Trauma. 1999;47(3 Suppl):S59–66. doi: 10.1097/00005373-199909001-00013. [DOI] [PubMed] [Google Scholar]
- 31.Nathens AB, Jurkovich GJ, Cummings P, Rivara FP, Maier RV. The effect of organized systems of trauma care on motor vehicle crash mortality. JAMA. 2000;283(15):1990–1994. doi: 10.1001/jama.283.15.1990. [DOI] [PubMed] [Google Scholar]
- 32.Hulka F, Mullins RJ, Mann NC, Hedges JR, Rowland D, Worrall WH, et al. Influence of a statewide trauma system on pediatric hospitalization and outcome. J Trauma. 1997;42(3):514–519. doi: 10.1097/00005373-199703000-00020. [DOI] [PubMed] [Google Scholar]
- 33.Rautava L, Lehtonen L, Peltola M, Korvenranta E, Korvenranta H, Linna M, et al. The effect of birth in secondary- or tertiary-level hospitals in Finland on mortality in very preterm infants: a birth-register study. Pediatrics. 2007;119(1):e257–263. doi: 10.1542/peds.2006-1964. [DOI] [PubMed] [Google Scholar]
- 34.Neto MT. Perinatal care in Portugal: effects of 15 years of a regionalized system. Acta Paediatr. 2006;95(11):1349–1352. doi: 10.1080/08035250600615135. [DOI] [PubMed] [Google Scholar]
- 35.Powell SL, Holt VL, Hickok DE, Easterling T, Connell FA. Recent changes in delivery site of low-birth-weight infants in Washington: Impact on birth weight-specific mortality. American Journal of Obstetrics and Gynecology. 1995;173(5):1585–1592. doi: 10.1016/0002-9378(95)90653-3. [DOI] [PubMed] [Google Scholar]
- 36.Bode MM, O’Shea TM, Metzguer KR, Stiles AD. Perinatal regionalization and neonatal mortality in North Carolina, 1968-1994. American Journal of Obstetrics and Gynecology. 2001;184(6):1302–1307. doi: 10.1067/mob.2001.114484. [DOI] [PubMed] [Google Scholar]
- 37.Yeast JD, Poskin M, Stockbauer JW, Shaffer S. Changing patterns in regionalization of perinatal care and the impact on neonatal mortality. American Journal of Obstetrics and Gynecology. 1998;178(1 Pt 1):131–135. doi: 10.1016/s0002-9378(98)70639-8. [DOI] [PubMed] [Google Scholar]
- 38.Shlossman PA, Manley JS, Sciscione AC, Colmorgen GH. An analysis of neonatal morbidity and mortality in maternal (in utero) and neonatal transports at 24-34 weeks’ gestation. Am J Perinatol. 1997;14(8):449–456. doi: 10.1055/s-2007-994178. [DOI] [PubMed] [Google Scholar]
- 39.Gould JB, Marks AR, Chavez G. Expansion of community-based perinatal care in California. J Perinatol. 2002;22(8):630–640. doi: 10.1038/sj.jp.7210824. [DOI] [PubMed] [Google Scholar]
- 40.Meadow W, Kim M, Mendez D, Bell A, Gray C, Corpuz M, et al. Which nurseries currently care for ventilated neonates in Illinois and Wisconsin? Implications for the next generation of perinatal regionalization. Am J Perinatol. 2002;19(4):197–203. doi: 10.1055/s-2002-28486. [DOI] [PubMed] [Google Scholar]
- 41.Nance ML, Carr BG, Branas CC. Access to pediatric trauma care in the United States. Arch Pediatr Adolesc Med. 2009;163(6):512–518. doi: 10.1001/archpediatrics.2009.65. [DOI] [PubMed] [Google Scholar]
- 42.McLean TR, Richards EP. Teleradiology: A case study of the economic and legal considerations in international trade in telemedicine. Health Aff. 2006;25(5):1378–1385. doi: 10.1377/hlthaff.25.5.1378. [DOI] [PubMed] [Google Scholar]
- 43.Silva RA, Murakami Y, Jain A, Gandhi J, Lad EM, Moshfeghi DM. Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP): 18-month experience with telemedicine screening. Graefes Arch Clin Exp Ophthalmol. 2009;247(1):129–136. doi: 10.1007/s00417-008-0943-z. [DOI] [PubMed] [Google Scholar]
- 44.Richter GM, Williams SL, Starren J, Flynn JT, Chiang MF. Telemedicine for retinopathy of prematurity diagnosis: Evaluation and challenges. Surv Ophthalmol. 2009;54(6):671–685. doi: 10.1016/j.survophthal.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwamm LH, Holloway RG, Amarenco P, Audebert HJ, Bakas T, Chumbler NR, et al. A review of the evidence for the use of telemedicine within stroke systems of care: A scientific statement from the American Heart Association/American Stroke Association. Stroke. 2009;40(7):2616–2634. doi: 10.1161/STROKEAHA.109.192360. [DOI] [PubMed] [Google Scholar]
- 46.Sapirstein A, Lone N, Latif A, Fackler J, Pronovost PJ. Tele ICU: Paradox or panacea? Best Pract Res Clin Anaesthesiol. 2009;23(1):115–126. doi: 10.1016/j.bpa.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Thomas EJ, Lucke JF, Wueste L, Weavind L, Patel B. Association of telemedicine for remote monitoring of intensive care patients with mortality, complications, and length of stay. JAMA. 2009;302(24):2671–2678. doi: 10.1001/jama.2009.1902. [DOI] [PubMed] [Google Scholar]
- 48.The photographic screening for retinopathy of prematurity study (photo-ROP). Primary outcomes. Retina. 2008;28(3 Suppl):S47–54. doi: 10.1097/IAE.0b013e31815e987f. Erratum in: Retina 2009 Jan;2029(2001):2127. [DOI] [PubMed] [Google Scholar]
- 49.Phibbs CS, Mark DH, Luft HS, Peltzman-Rennie DJ, Garnick DW, Lichtenberg E, et al. Choice of hospital for delivery: A comparison of high-risk and low-risk women. Health Serv Res. 1993;28(2):201–222. [PMC free article] [PubMed] [Google Scholar]
- 50.Finlayson SR, Birkmeyer JD, Tosteson AN, Nease RF., Jr Patient preferences for location of care: Implications for regionalization. Med Care. 1999;37(2):204–209. doi: 10.1097/00005650-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Kansagra SM, Curtis LH, Schulman KA. Regionalization of percutaneous transluminal coronary angioplasty and implications for patient travel distance. JAMA. 2004;292(14):1717–1723. doi: 10.1001/jama.292.14.1717. [DOI] [PubMed] [Google Scholar]
- 52.Birkmeyer JD, Siewers AE, Marth NJ, Goodman DC. Regionalization of high-risk surgery and implications for patient travel times. JAMA. 2003;290(20):2703–2708. doi: 10.1001/jama.290.20.2703. [DOI] [PubMed] [Google Scholar]
- 53.Donohue PK, Hussey-Gardner B, Sulpar LJ, Fox R, Aucott SW. Parents’ perception of the back-transport of very-low-birth-weight infants to community hospitals. J Perinatol. 2009;29(8):575–581. doi: 10.1038/jp.2009.17. [DOI] [PubMed] [Google Scholar]
- 54.Hanrahan K, Gates M, Attar MA, Lang SW, Frohna A, Clark SJ. Neonatal back transport: Perspectives from parents of Medicaid-insured infants and providers. Neonatal Netw. 2007;26(5):301–311. doi: 10.1891/0730-0832.26.5.301. [DOI] [PubMed] [Google Scholar]
- 55.Attar MA, Lang SW, Gates MR, Iatrow AM, Bratton SL. Back transport of neonates: Effect on hospital length of stay. J Perinatol. 2005;25(11):731–736. doi: 10.1038/sj.jp.7211391. [DOI] [PubMed] [Google Scholar]
- 56.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. Journal of pediatric gastroenterology and nutrition. 2001;138:92–100. doi: 10.1067/mpd.2001.109608. [DOI] [PubMed] [Google Scholar]
- 57.Semmlow JL, Cone R. Utility of the injury severity score: A confirmation. Health Serv Res. 1976;11(1):45–52. [PMC free article] [PubMed] [Google Scholar]
- 58.Boyd CR, Tolson MA, Copes WS. Evaluating trauma care: The TRISS method. Trauma Score and the Injury Severity Score. J Trauma. 1987;27(4):370–378. [PubMed] [Google Scholar]
- 59.Pillgram-Larsen J, Marcus M, Svennevig JL. Assessment of probability of survival in penetrating injuries using the TRISS methodology. Injury. 1989;20(1):10–12. doi: 10.1016/0020-1383(89)90035-1. [DOI] [PubMed] [Google Scholar]
- 60.Durbin DR, Localio AR, MacKenzie EJ. Validation of the ICD/AIS MAP for pediatric use. Inj Prev. 2001;7(2):96–99. doi: 10.1136/ip.7.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kollee LA, Verloove-Vanhorick PP, Verwey RA, Brand R, Ruys JH. Maternal and neonatal transport: Results of a national collaborative survey of preterm and very low birth weight infants in The Netherlands. Obstet Gynecol. 1988;72(5):729–732. [PubMed] [Google Scholar]
- 62.Mayfield JA, Rosenblatt RA, Baldwin LM, Chu J, Logerfo JP. The relation of obstetrical volume and nursery level to perinatal mortality. Am J Public Health. 1990;80(7):819–823. doi: 10.2105/ajph.80.7.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moore L, Lavoie A, Turgeon AF, Abdous B, Le Sage N, Emond M, et al. The trauma risk adjustment model: A new model for evaluating trauma care. Ann Surg. 2009;249(6):1040–1046. doi: 10.1097/SLA.0b013e3181a6cd97. [DOI] [PubMed] [Google Scholar]
- 64.Demetriades D, Chan LS, Velmahos G, Berne TV, Cornwell EE, 3rd, Belzberg H, et al. TRISS methodology in trauma: The need for alternatives. Br J Surg. 1998;85(3):379–384. doi: 10.1046/j.1365-2168.1998.00610.x. [DOI] [PubMed] [Google Scholar]
- 65.Demetriades D, Chan L, Velmanos GV, Sava J, Preston C, Gruzinski G, et al. TRISS methodology: An inappropriate tool for comparing outcomes between trauma centers. J Am Coll Surg. 2001;193(3):250–254. doi: 10.1016/s1072-7515(01)00993-0. [DOI] [PubMed] [Google Scholar]
- 66.Cayten CG, Stahl WM, Murphy JG, Agarwal N, Byrne DW. Limitations of the TRISS method for interhospital comparisons: a multihospital study. J Trauma. 1991;31(4):471–481. doi: 10.1097/00005373-199104000-00005. discussion 481-472. [DOI] [PubMed] [Google Scholar]
- 67.Gabbe BJ, Cameron PA, Wolfe R. TRISS: Does it get better than this? Acad Emerg Med. 2004;11(2):181–186. [PubMed] [Google Scholar]
- 68.Schall LC, Potoka DA, Ford HR. A new method for estimating probability of survival in pediatric patients using revised TRISS methodology based on age-adjusted weights. J Trauma. 2002;52(2):235–241. doi: 10.1097/00005373-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention. Field Triage Decision Scheme. 2009 Available at: < http://www.cdc.gov/fieldtriage/>. Published.
- 70.Vandenbroucke JP. When are observational studies as credible as randomised trials? Lancet. 2004;363(9422):1728–1731. doi: 10.1016/S0140-6736(04)16261-2. [DOI] [PubMed] [Google Scholar]
- 71.Little RJ, Rubin DB. Causal effects in clinical and epidemiological studies via potential outcomes: Concepts and analytical approaches. Annu Rev Public Health. 2000;21:121–145. doi: 10.1146/annurev.publhealth.21.1.121. [DOI] [PubMed] [Google Scholar]
- 72.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8 Pt 2):757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 73.Joffe MM, Rosenbaum PR. Invited commentary: Propensity scores. Am J Epidemiol. 1999;150(4):327–333. doi: 10.1093/oxfordjournals.aje.a010011. [DOI] [PubMed] [Google Scholar]
- 74.Newhouse JP, McClellan M. Econometrics in outcomes research: The use of instrumental variables. Annu Rev Public Health. 1998;19:17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 75.Horbar JD, Badger GJ, Lewit EM, Rogowski J, Shiono PH. Hospital and patient characteristics associated with variation in 28-day mortality rates for very low birth weight infants. Vermont Oxford Network. Pediatrics. 1997;99(2):149–156. doi: 10.1542/peds.99.2.149. [DOI] [PubMed] [Google Scholar]
- 76.Sankaran K, Chien LY, Walker R, Seshia M, Ohlsson A, Canadian Neonatal N. Variations in mortality rates among Canadian neonatal intensive care units. Can Med Assoc J. 2002;166(2):173–178. [PMC free article] [PubMed] [Google Scholar]
- 77.Clark SJ, Yoxall CW, Subhedar NV. Right ventricular performance in hypotensive preterm neonates treated with dopamine. Pediatr Cardiol. 2002;23(2):167–170. doi: 10.1007/s00246-001-0041-z. [DOI] [PubMed] [Google Scholar]
- 78.Siegel E, Gillings D, Campbell S, Guild P. Controlled evaluation of rural regional perinatal care: Developmental and neurologic outcomes at 1 year. Pediatrics. 1986;77(2):187–195. [PubMed] [Google Scholar]
- 79.Kollee LA, Brand R, Schreuder AM, Ens-Dokkum MH, Veen S, Verloove-Vanhorick SP. Five-year outcome of preterm and very low birth weight infants: A comparison between maternal and neonatal transport. Obstet Gynecol. 1992;80(4):635–638. [PubMed] [Google Scholar]
- 80.Potoka DA, Schall LC, Ford HR. Improved functional outcome for severely injured children treated at pediatric trauma centers. J Trauma. 2001;51(5):824–832. doi: 10.1097/00005373-200111000-00002. discussion 832-824. [DOI] [PubMed] [Google Scholar]
- 81.Synnes AR, Macnab YC, Qiu Z, Ohlsson A, Gustafson P, Dean CB, et al. Neonatal intensive care unit characteristics affect the incidence of severe intraventricular hemorrhage. Med Care. 2006;44(8):754–759. doi: 10.1097/01.mlr.0000218780.16064.df. [DOI] [PubMed] [Google Scholar]
- 82.Keller MS, Vane DW. Management of pediatric blunt splenic injury: Comparison of pediatric and adult trauma surgeons. J Pediatr Surg. 1995;30(2):221–224. doi: 10.1016/0022-3468(95)90564-2. discussion 224-225. [DOI] [PubMed] [Google Scholar]
- 83.Frumiento C, Vane DW. Changing patterns of treatment for blunt splenic injuries: An 11-year experience in a rural state. J Pediatr Surg. 2000;35(6):985–988. doi: 10.1053/jpsu.2000.6948. discussion 988-989. [DOI] [PubMed] [Google Scholar]
- 84.Mooney DP, Birkmeyer NJ, Udell JV, Shorter NA. Variation in the management of pediatric splenic injuries in New Hampshire. J Pediatr Surg. 1998;33(7):1076–1078. doi: 10.1016/s0022-3468(98)90534-6. discussion 1079-1080. [DOI] [PubMed] [Google Scholar]
- 85.Jacobs IA, Kelly K, Valenziano C, Pawar J, Jones C. Nonoperative management of blunt splenic and hepatic trauma in the pediatric population: Significant differences between adult and pediatric surgeons? Am Surg. 2001;67(2):149–154. [PubMed] [Google Scholar]
- 86.Myers JG, Dent DL, Stewart RM, Gray GA, Smith DS, Rhodes JE, et al. Blunt splenic injuries: Dedicated trauma surgeons can achieve a high rate of nonoperative success in patients of all ages. J Trauma. 2000;48(5):801–805. doi: 10.1097/00005373-200005000-00002. discussion 805-806. [DOI] [PubMed] [Google Scholar]
- 87.Rogowski JA, Horbar JD, Staiger DO, Kenny M, Carpenter J, Geppert J. Indirect vs direct hospital quality indicators for very low-birth-weight infants. JAMA. 2004;291(2):202–209. doi: 10.1001/jama.291.2.202. [DOI] [PubMed] [Google Scholar]
- 88.Rogowski JA, Staiger DO, Horbar JD. Variations in the quality of care for very-low-birthweight infants: implications for policy. Health Aff. 2004;23(5):88–97. doi: 10.1377/hlthaff.23.5.88. [DOI] [PubMed] [Google Scholar]
- 89.Shafi S, Friese R, Gentilello LM. Moving beyond personnel and process: A case for incorporating outcome measures in the trauma center designation process. Arch Surg. 2008;143(2):115–119. doi: 10.1001/archsurg.2007.29. discussion 120. [DOI] [PubMed] [Google Scholar]
- 90.Profit J, Zupancic JA, Gould JB, Petersen LA. Implementing pay-for-performance in the neonatal intensive care unit. Pediatrics. 2007;119(5):975–982. doi: 10.1542/peds.2006-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]


