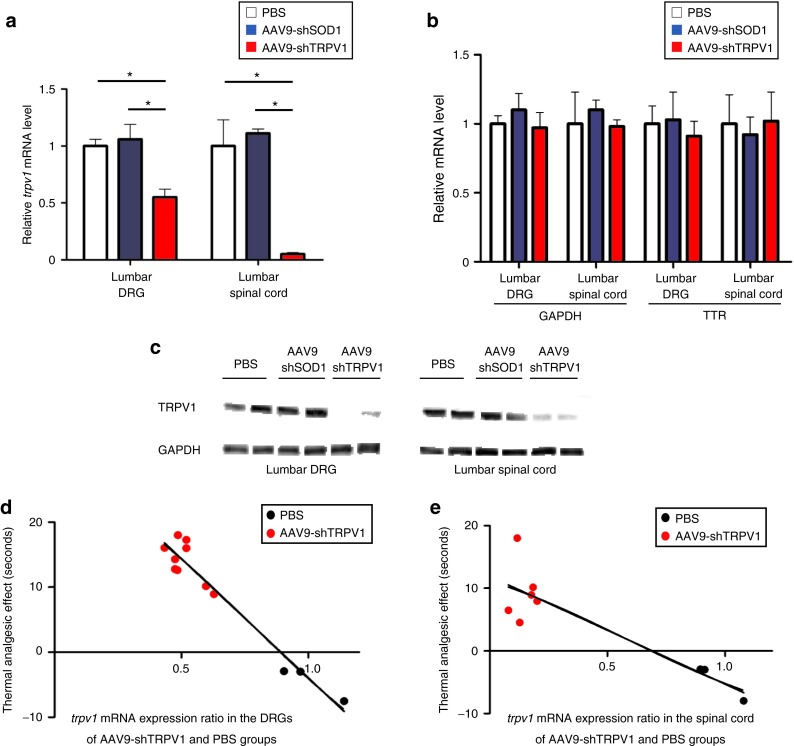
Figure 4.
Intrathecal administration of AAV9-shTRPV1 specifically suppresses trpv1 mRNA and TRPV1 protein expression in vivo. (a) Quantitative reverse transcription polymerase chain reaction (RT-PCR) analysis of trpv1 mRNA expression in lumbar dorsal root ganglia (DRG) and spinal cord in mice 4 weeks after intrathecal injection of phosphate-buffered saline (PBS) (control), AAV9-shSOD1 or AAV9-shTRPV1. trpv1 mRNA expression was significantly (*P < 0.05) inhibited in the lumbar DRG and spinal cord of the AAV9-shTRPV1-treated group relative to the two other groups. (b) Quantitative RT-PCR analysis of the expression of non-targeted ttr and gapdh mRNA in the lumbar DRG and spinal cord at 4 weeks after the treatment. No significant differences were found in the expression of ttr and gapdh mRNA among the three groups. Data are presented as means ± SEM (n = 4 or 5 mice per group; *P < 0.05). (c) TRPV1 protein levels in the lumbar DRG and spinal cord of two mice from each group, as assessed by Western blot analysis 4 weeks after treatment. A robust reduction of TRPV1 protein levels in both the DRG and spinal cord was observed in the AAV9-shTRPV1-treated group. (d) and (e) Relationship between thermal analgesia and trpv1 mRNA expression in the lumbar DRG and spinal cord. Analgesia in response to 50 °C thermal stimulation was significantly correlated with trpv1 mRNA expression in the lumbar DRG (Y = −36.64X+32.70; R2: 0.94, P < 0.01) and spinal cord (e, Y = −17.2X+11.91; R2: 0.79, P < 0.01).