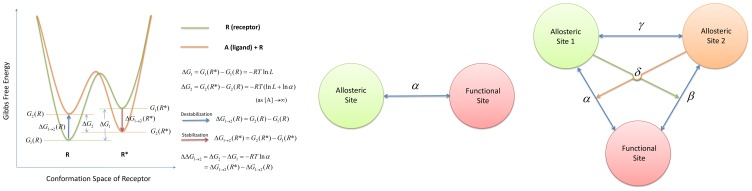
Figure 5. The thermodynamic and free energy landscape of the population shift views, the structural view of the allosteric two-state model, and an extension of the model to two allosteric sites and one functional site.
(A) The free energy landscape presentation of ATSM. Before binding, the relative free energy between the inactive ( ) and active (
) and active ( ) states is given by
) states is given by 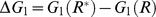 , which is
, which is 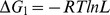 according to the ATSM as depicted by the light green curve. After binding, the relative free energy between
according to the ATSM as depicted by the light green curve. After binding, the relative free energy between 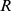 and
and 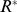 is given by
is given by 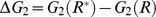 , which under a saturating ligand concentration becomes
, which under a saturating ligand concentration becomes 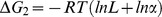 , as drawn by the orange curve. The extent of population shift as measured by the free energy change due to binding,
, as drawn by the orange curve. The extent of population shift as measured by the free energy change due to binding, 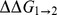 , is equal to
, is equal to 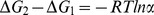 . This result implies that the allosteric effect is solely determined by the allosteric efficacy, α, but not the absolute ligand affinity.
. This result implies that the allosteric effect is solely determined by the allosteric efficacy, α, but not the absolute ligand affinity. 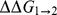 can also be expressed by the difference between the active conformation stabilization energy,
can also be expressed by the difference between the active conformation stabilization energy, 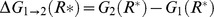 (red arrow), and inactive conformation destabilization energy,
(red arrow), and inactive conformation destabilization energy, 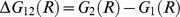 (blue arrow). (B) The structural view of allostery according to the ATSM. The allosteric communication between the allosteric and functional sites is indicated by the arrow with the coupling specified by the allosteric efficacy
(blue arrow). (B) The structural view of allostery according to the ATSM. The allosteric communication between the allosteric and functional sites is indicated by the arrow with the coupling specified by the allosteric efficacy  . Unlike the thermodynamic view, the structural view emphasizes that the conformations of two sites breathe dynamically in a concerted motion through a set of mutually interacting residues. Without such a propagation channel between sites,
. Unlike the thermodynamic view, the structural view emphasizes that the conformations of two sites breathe dynamically in a concerted motion through a set of mutually interacting residues. Without such a propagation channel between sites, 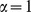 is always the case, no matter the changes at the allosteric site. Thus, while a preexisting channel (or allosteric networks of correlated residues) is a required condition, by itself the communication through the channel does not determine the allosteric efficacy. (C) The structural view of allostery according to the extended ATSM. In the drawing, the two allosteric communication channels between the two allosteric sites and the functional site are indicated by the blue double arrows with the coupling specified by the allosteric efficacy
is always the case, no matter the changes at the allosteric site. Thus, while a preexisting channel (or allosteric networks of correlated residues) is a required condition, by itself the communication through the channel does not determine the allosteric efficacy. (C) The structural view of allostery according to the extended ATSM. In the drawing, the two allosteric communication channels between the two allosteric sites and the functional site are indicated by the blue double arrows with the coupling specified by the allosteric efficacy  ,
, 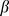 from the extended ATSM. The communication between the two allosteric sites is linked with a coupling specified by the binding cooperativity,
from the extended ATSM. The communication between the two allosteric sites is linked with a coupling specified by the binding cooperativity,  , which is shown not to affect the allosteric efficacy directly. The activation cooperativity
, which is shown not to affect the allosteric efficacy directly. The activation cooperativity  is the sum of the allosteric effect of site 1 toward coupling
is the sum of the allosteric effect of site 1 toward coupling 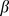 (pale green arrow) plus allosteric site 2 toward allosteric coupling α (orange arrow). As in the simplest ATSM, it is the ligand binding itself that puts forth the allosteric communications through existing propagation channels and determines the allosteric efficacy and the activation cooperativity either positively or negatively.
(pale green arrow) plus allosteric site 2 toward allosteric coupling α (orange arrow). As in the simplest ATSM, it is the ligand binding itself that puts forth the allosteric communications through existing propagation channels and determines the allosteric efficacy and the activation cooperativity either positively or negatively.