Abstract
Background
Adolescent idiopathic scoliosis (AIS) is associated with low bone mineral density (BMD). The underlying etiology and how it may relate to the development of osteopenia remains unknown. Leptin has been postulated as one of the etiologic factors of AIS because of its profound effects on bone metabolism and pubertal growth. Its modulator, soluble leptin receptor (sOB-R), may affect leptin bioavailability and signaling. This study aimed to investigate whether serum leptin and sOB-R levels may be associated with bone quality, and whether these relationships may differ between young adolescent girls with and without AIS.
Methods
This was a case-control study involving 94 newly diagnosed AIS girls (Cobb angle 12–48°) aged 12 to 14 years old and 87 age and gender-matched normal controls. Subjects with BMI>23.0 Kg/m2 were excluded. Anthropometric measurements including body weight, height, arm span and sitting height were taken. Serum total leptin and sOB-R were assayed with ELISA. Non-dominant distal radius was scanned with High Resolution pQCT for assessing bone quality in terms of bone morphometry, volumetric BMD (vBMD) and trabecular bone micro-architecture.
Results
Compared with normal controls, AIS girls had numerically higher sOB-R (p = 0.006), lower average vBMD (p = 0.048), lower cortical vBMD (p = 0.029), higher cortical bone perimeter (p = 0.014) and higher trabecular area (p = 0.027), but none remained statistically significant after the Hochberg-Benjamini procedure. Correlation analysis on serum leptin level indicated that distinctive correlations with trabecular bone parameters occurred only in AIS.
Conclusion
This study showed that bone quality in AIS girls was deranged as compared with controls. In addition, the distinct differences in correlation pattern between leptin and trabecular bone parameters indicated possible abnormalities in bone metabolism and dysfunction of the leptin signaling pathway in AIS.
Introduction
Adolescent Idiopathic Scoliosis (AIS) is a complex three-dimensional spinal deformity affecting mainly girls with disease onset at the peripubertal period [1], [2]. AIS can be a serious condition carrying significant morbidities especially for patients with severe curves. It is important to elucidate the etiopathogenesis of AIS so that effective preventive and therapeutic measures can be devised. Previous studies have reported the association between AIS and low body weight, tall stature, increased arm span, low body mass index, delayed menarche and low bone mass [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. Special attention is paid to low bone mass as Hung et al. [16] showed that it was a significant prognostic indicator for curve progression. What mediates these observed characteristic anthropometric phenotypes, delayed pubertal development, abnormal skeletal growth and disturbed bone mineral homeostasis in AIS remains unresolved.
Leptin, a 16 kDa protein hormone predominantly secreted by adipocytes, is known to be an important factor in neuro-osseous development affecting skeletal growth, the onset of puberty, energy expenditure and body composition [17], [18], [19], [20], [21], [22], [23]. Leptin actions are mediated through leptin receptors expressed in the hypothalamus and peripheral tissues [24], [25], [26], [27], [28], [29], [30]. The peripheral effects of leptin have been shown to be directly anabolic to bone formation [31], promoting the proliferation of osteoblasts [32] and chondrocytes [33], stimulating osteoblastic differentiation [32], [34], mineralization [29], [32], [35] and inhibiting osteoclastogenesis and osteoclast activity [32], [36], [37]. On the other hand, leptin have also been shown to exert a catabolic effect on bone formation through the hypothalamus via the sympathetic nervous system (SNS) [38], [39], [40], [41].
AIS and higher levels of serum leptin are found more commonly in girls versus boys, suggesting that leptin may play an important role in the pubertal growth of girls [42], [43]. The abnormal phenotypic profiles that characterize AIS girls including low body weight, tall stature, increased arm span, low body mass index, delayed menarche and low bone mass also happen to coincide with leptin’s physiological effects [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. It was thus logical to investigate the possible role of leptin in the etiopathogenesis of AIS. A previous study [44] has shown that children with lower BMI have more severe truncal asymmetry. Girls with lower BMI likely have lower leptin levels that could be a potential cause of AIS. In mice, Wu et al. [45] reported that high central leptin activity might increase the risk of developing scoliosis. In a previous study conducted by our joint center, significantly lower serum leptin levels were found in AIS girls without controlling for the BMI status [46]. Subsequent studies by our group documented increased serum levels of sOB-R in AIS as compared with matched normal controls [15]. It is possible that changes in the sOB-R level might alter the bioavailability of leptin at the cellular and tissue levels, resulting in the abnormal phenotypic expression that was observed in AIS girls.
To the best of our knowledge, bone quality assessment with HR-pQCT and its correlation with leptin bioavailability has not been reported in the literature. The purpose of the current study aimed at testing the hypothesis that 3-dimensional bone quality parameters and its correlations with serum total leptin and sOB-R levels are distinct in AIS when compared with normal matched controls.
Materials and Methods
Ethics Statement
Ethics approval for the present study was obtained from the Joint Chinese University of Hong Kong - New Territories East Cluster Clinical Research Ethics Committee (CREC), Hong Kong (CRE 2010.066). Informed written consents were obtained from all the subjects as well as from their legal guardians before enrollment into the study.
Subjects Recruitment
94 Chinese AIS girls aged between 12 and 14 years old without prior treatment were recruited consecutively from our scoliosis clinic. 87 age-matched healthy girls were recruited from local schools. Subjects with BMI>23.0 were excluded to avoid bias from the relatively high serum leptin levels known to be present in overweight/obese subjects [47], [48]. The diagnosis for AIS (Cobb angle ≥10°) was confirmed with detailed clinical and radiological assessment and the standard standing antero-posterior radiographs were used to assess curve severity. Curve types of the AIS girls were recorded according to the Lenke Classification [49]. All control subjects were examined by an experienced orthopaedic surgeon (Dr. T.P. Lam) to rule out spinal deformities. Subjects with history of congenital deformities, neuromuscular diseases, skeletal dysplasia, endocrine diseases, connective tissue abnormalities, mental retardation, or history of recent steroid intake were excluded from the study.
Anthropometric Assessment
Anthropometric parameters including body height, body weight, sitting height and arm span were measured. Height was recorded without shoes, standing against a wall-mounted stadiometer and measurement was taken to the nearest 0.1 cm. Weight was measured to the nearest 0.1 kg in light clothes without shoes. Sitting height was measured by a stadiometer, the subjects were asked to sit straight and measurement was taken to the nearest 0.1 cm. Arm span was measured by using a wall-mounted tape to the nearest 0.1 cm. As shown by previous studies [50], [51], the different equations for correction of height loss resulted from the spinal deformity had limitation and might not fit every curve. Moreover, it has been shown that linear correlation between arm span and standing height in healthy children and adolescents is very high (r2 = 0.99) [52]. Hence, arm span was used for calculating the BMI in place of body height in the current study (body weight/armspan2).
Assessment of Bone Quality with High Resolution pQCT
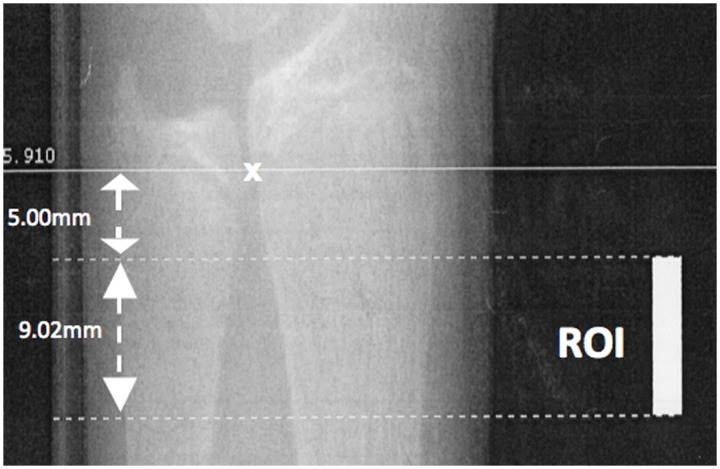
The non-dominant distal radius was measured by HR-pQCT (XtremeCT, Scanco Medical, Brüttisellen, Switzerland) according to the standard protocol [53]. Subjects were instructed to put their forearm in a customized cast and hold the handle of the cast to minimize the variation of rotation between and within subjects. Using a scout view, a reference line was placed at the most proximal limit of the inner aspect of the epiphyseal growth plate as shown in Figure 1. The scan was performed on a segment spanning 9.02 mm starting from 5 mm proximal to the reference line. 110 CT slices with a nominal resolution (voxel size) of 82 µm were obtained using the following settings: a microfocus X-Ray-source of 60 kVp and a matrix size of 1536×1536. The effective dose was less than 5 µSv and the measurement time was 3 minutes. Distal radius measurements using HR-pQCT were found to correlate well with systemic BMD status in premenopausal women [54], and combined with its low radiation and avoidance of weight bearing effect on BMD, it represented a favorable bone imaging technique for our adolescent subjects.
Figure 1. Representative scout view of the distal radius scanned with high resolution peripheral quantitative computed tomography.
The reference line is marked at the most proximal point of the inner aspect of the growth plate (marked with x). The region of interest (ROI) spanning 9.02 mm starts from 5 mm proximal to the reference line. 110 CT slices with a nominal resolution (voxel size) of 82 µm were obtained.
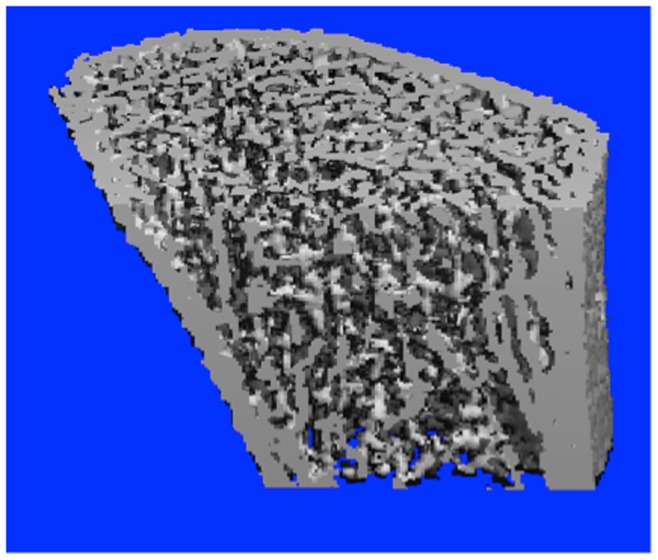
Methods used to process HR-pQCT data have been described in details by Laib et al. [55]. A typical reconstructed image was shown in Figure 2. HR-pQCT bone parameters were divided into three categories namely A) volumetric bone mineral density (vBMD), B) bone morphometry, and C) trabecular bone micro-architecture.
Figure 2. Three-dimensional HR-pQCT image of the distal radius with crosscut showing the trabecular bone.
A) Volumetric BMD
Average vBMD, Cortical Bone vBMD and Trabecular Bone vBMD in milligram of hydroxyapatite per cubic centimeter (mgHA/cm3) were computed as the average mineral density of the total, cortical and trabecular volume of interest, respectively. The CV% for various vBMDs ranged from 1.27% to 2.21% for the study population.
B) Bone morphometry
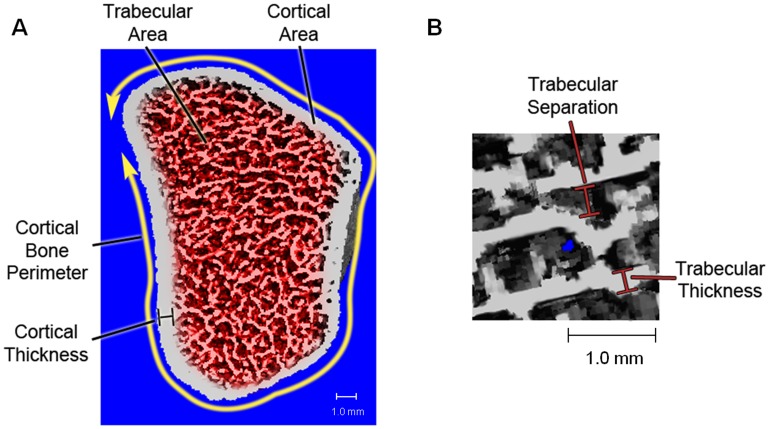
The focused zone was automatically separated into cortical and trabecular compartments using a Gaussian filter and a threshold-based algorithm. The threshold used to discriminate the cortical from trabecular bone was set to one third of the apparent cortical bone density as previously described by Laib et al. [55]. Trabecular Area and Cortical Area were measured directly. Cortical Bone Perimeter was calculated from the contour. Mean Cortical Thickness was defined as the mean cortical volume divided by the outer bone surface. Illustration of these parameters is shown in Figure 3A. Excellent correlation (r = 0.98) has been shown for Cortical Thickness measurement compared with the gold standard of ex-vivo microCT data [56]. The coefficient of variation % (CV%) ranged from 1.35%–6.58%.
Figure 3. Illustrations of A) bone morphometry parameters and B) trabecular separation and thickness.
C) Trabecular bone micro-architecture
Trabecular Bone Volume to Tissue Volume Ratio (BV/TV) was derived from Trabecular Bone vBMD assuming mineral density of fully mineralized bone of 1200 mg hydroxyapatite [i.e. Trabecular Bone vBMD (mgHA/cm3)/1200 mgHA/cm3]. The spatial resolution of the resulting images from HR-pQCT is not sufficient to depict individual trabeculae, micro-architecture parameters were extracted with the help of a ridge detection algorithm described previously by Laib et al. [55], [57] and Hildebrand and Rüegsegger [58]. Trabecular Number (mm−1) was defined as the inverse of the mean spacing of the ridges [55], [57] and thus a truly three-dimensional data is evaluated independent of the plate or rod-like nature of the trabeculae structure. Trabecular Thickness (mm) and Trabecular Separation (mm) were derived from BV/TV and Trabecular Number using standard histomorphometry and by the following formulae: Trabecular Thickness = (BV/TV)/Trabecular Number and Trabecular Separation = (1-BV/TV)/Trabecular Number. Illustration of the physical meanings of these parameters is shown in Figure 3B. In the validation studies, excellent correlations (r≥0.96) have been shown for the values of these trabecular bone micro-architecture parameters when compared with the microCT measurements [59]. The CV% for the various parameters ranged from 1.25% to 4.65%.
Biochemical Assays
Non-fasting peripheral venous blood samples were collected from the subjects, serum was separated and stored at −80°C until analysis. Serum total leptin levels were measured by enzyme-linked immunosorbent assay (ELISA) specific for human leptin (ALPCO Diagnostics, Salem, NH, USA) following a standard protocol. The lowest detection limit for leptin was 0.50 ng/ml. Serum sOB-R levels were measured by ELISA (R&D systems, Minneapolis, USA). The minimum detectable concentration was 0.057 ng/ml.
Definition of Terms
For sake of clarity, terminology on leptin presented in this report is defined as follows:
Soluble leptin receptor (sOB-R) – main leptin binding protein in circulation, not a signalling receptor
Leptin receptor (Ob-R) – membrane bound signalling/transport receptor for free leptin
Free leptin – active form of leptin that could be bound to leptin receptor
Bound leptin – leptin that is reversibly bound to sOB-R in the circulation
Serum total leptin – free leptin+bound leptin in serum
Leptin bioavailability – amount of leptin that is available to leptin receptor on target tissues for signal transduction
Statistical Analysis
Data are presented as mean ± SD. Anthropometric data, bone quality data, serum total leptin levels, and sOB-R levels were compared between AIS and normal controls using independent samples t-test. Bone quality measurement by HR-pQCT was separated into cortical bone parameters (cortical vBMD, cortical area, cortical thickness, and cortical bone perimeter) and trabecular bone parameters (trabecular vBMD, trabecular area, trabecular BV/TV, trabecular number, trabecular thickness, and trabecular separation) for correlation analysis. Multiple regression analysis was used to analyze differences in bone quality parameters between groups with adjustment of age and BMI. The correlations between bone quality parameters and serum total leptin and sOB-R of the AIS and control group were analyzed with Pearson’s correlation analysis and multiple regression analysis with age and BMI adjustment. To correct for multiple comparisons, false discovery rate (FDR) adjusted p-value derived from the Hochberg-Benjamini procedure was determined. SPSS 16.0 for Windows (SPSS Inc., Chicago, IL) was used for statistical analysis. FDR-adjusted p<0.05 was considered statistically significant.
Results
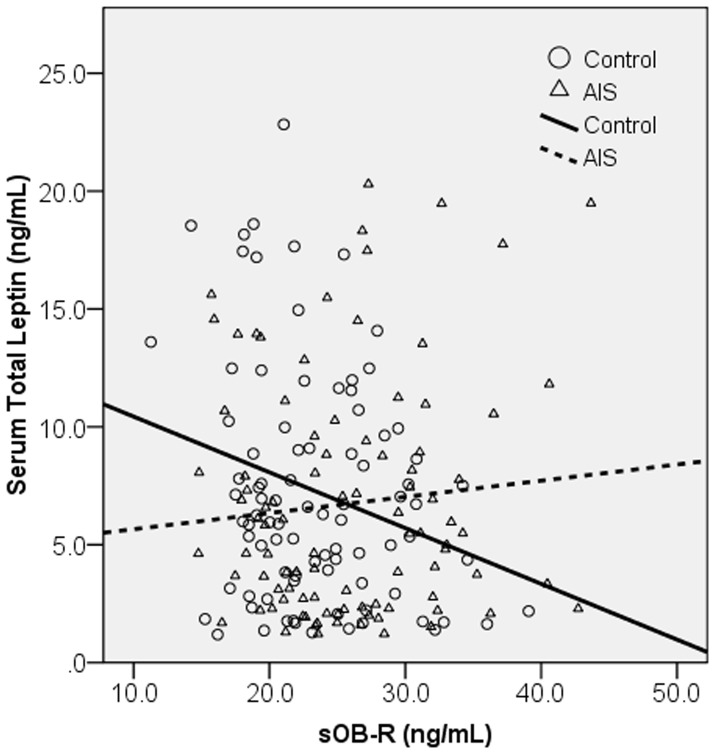
The mean Cobb angle of AIS girls was 25.6±6.7° (range 12–48°). 97.9% of the AIS girls were right handed. According to Lenke Classification, 17.2% of the AIS girls were type 1, 4.3% type 2, 36.6% type 3, 6.5% type 4, 19.4% type 5, and 16.1% type 6. A total of 51.1% of the AIS girls have right thoracic curve component. AIS girls had lower BMI (17.91±2.20 vs. 18.64±2.15 kg/m2, p = 0.024), and higher sOB-R (25.86±6.49 vs. 23.43±5.27 ng/mL, p = 0.006) than normal controls (Table 1). Normality test with normal probability plot showed that serum total leptin and sOB-R were fairly normally distributed in both AIS and control groups (results not shown). Age, body weight, body height, arm span, sitting height, tanner stages and serum total leptin were similar between the two groups (Table 1). Controls showed a statistically significant negative correlation between serum total leptin and sOB-R (r = −0.250, p = 0.020), while the correlation could not be detected for AIS subjects (r = 0.089, p = 0.394) (Figure 4).
Table 1. Anthropometrics, serum total leptin and sOB-R levels in controls and AIS girls.
| Controls | AIS | |||||
| Mean | SD | Mean | SD | % Difference | p-value | |
| Age | 12.93 | 0.43 | 13.05 | 0.52 | 0.96 | 0.081 |
| Weight (kg) | 43.79 | 6.71 | 42.94 | 6.42 | −1.93 | 0.387 |
| Height (cm) | 154.58 | 6.64 | 155.24 | 5.62 | 0.43 | 0.472 |
| Arm Span (cm) | 153.01 | 7.43 | 154.72 | 6.70 | 1.12 | 0.105 |
| Sitting Height (cm) | 82.04 | 3.67 | 82.30 | 3.56 | 0.32 | 0.638 |
| BMI (Kg/m2)** | 18.64 | 2.15 | 17.91 | 2.20 | −3.92 | 0.024* |
| Tanner Stage (breast) | 3.02 | 0.71 | 3.14 | 0.80 | 3.97 | 0.309 |
| Tanner Stage (pubic hair) | 2.45 | 0.83 | 2.52 | 0.77 | 2.86 | 0.541 |
| Leptin (ng/mL) | 7.26 | 4.99 | 6.75 | 5.01 | −7.03 | 0.493 |
| sOB-R (ng/mL) | 23.43 | 5.27 | 25.86 | 6.49 | 10.37 | 0.006* |
*p-value <0.05.
**BMI by arm span (body weight/armspan2).
Abbreviations: AIS, adolescent idiopathic scoliosis; sOB-R, soluble leptin receptor.
Figure 4. Correlation between serum total leptin and soluble leptin receptor (sOB-R) levels in AIS and controls.
Controls showed a significant negative correlation between serum total leptin and sOB-R (r = −0.250, p = 0.020), while AIS showed no correlation (r = 0.089, p = 0.394).
AIS girls had numerically lower average vBMD (256.2±51.4 vs. 270.9±45.6 mgHA/cm3, age and BMI adjusted p = 0.048, FDR-adjusted p = 0.132), and numerically lower cortical vBMD (688.4±79.0 vs. 712.6±62.5 mgHA/cm3, age and BMI adjusted p = 0.029, FDR-adjusted p = 0.106). Trabecular vBMD was numerically lower in AIS than in controls, although the difference did not reach statistical significance (Table 2). In bone morphometry, AIS girls have numerically higher cortical bone perimeter (55.03±4.14 vs. 53.56±4.50 mm, age and BMI adjusted p = 0.014, FDR-adjusted p = 0.106), and numerically higher trabecular area (149.1±27.5 vs. 140.0±27.3 mm2, age and BMI adjusted p = 0.027, FDR-adjusted p = 0.106). Trabecular BV/TV, trabecular number and trabecular thickness were numerically lower and trabecular separation was numerically higher in AIS, although the differences did not reach statistical significance (Table 2).
Table 2. Comparison of bone quality parameters between controls and AIS.
| Controls | AIS | ||||||
| Mean | SD | Mean | SD | % Diff | p-value | Age & BMI adjusted p-value | |
| A) Volumetric BMD | |||||||
| Average Bone Mineral Density (mgHA/cm3) | 270.9 | 45.6 | 256.2 | 51.4 | −5.43 | 0.044* | 0.048* |
| Cortical Bone Mineral Density (mgHA/cm3) | 712.6 | 62.5 | 688.4 | 79.0 | −3.39 | 0.023* | 0.029* |
| Trabecular Bone Mineral Density (mgHA/cm3) | 154.1 | 26.7 | 149.0 | 25.7 | −3.31 | 0.193 | 0.292 |
| B) Bone Morphometry | |||||||
| Cortical Area (mm2) | 28.1 | 10.7 | 25.8 | 12.3 | −8.47 | 0.168 | 0.319 |
| Cortical Thickness (mm) | 0.53 | 0.20 | 0.47 | 0.23 | −10.22 | 0.091 | 0.178 |
| Cortical Bone Perimeter (mm) | 53.56 | 4.50 | 55.03 | 4.14 | 2.74 | 0.024* | 0.014* |
| Trabecular Area (mm2) | 140.0 | 27.3 | 149.1 | 27.5 | 6.52 | 0.026* | 0.027* |
| C) Trabecular Bone Micro-architecture | |||||||
| Trabecular BV/TV | 0.128 | 0.022 | 0.124 | 0.021 | −3.25 | 0.200 | 0.303 |
| Trabecular Number (mm−1) | 1.766 | 0.225 | 1.728 | 0.203 | −2.18 | 0.228 | 0.360 |
| Trabecular Thickness (mm) | 0.073 | 0.007 | 0.072 | 0.009 | −0.90 | 0.599 | 0.625 |
| Trabecular Separation (mm) | 0.503 | 0.074 | 0.515 | 0.071 | 2.44 | 0.256 | 0.398 |
* p-value <0.05.
Abbreviations: AIS, adolescent idiopathic scoliosis; BMD, bone mineral density; BV/TV, bone volume to tissue volume ratio; Diff, difference; HA, hydroxyapatite.
Results of correlation analysis with cortical bone parameters were shown in Table 3. With Pearson’s correlation analysis, controls had significant correlations between serum total leptin and all cortical bone parameters (cortical vBMD (r = 0.287), cortical area (r = 0.294), cortical thickness (r = 0.249) and cortical bone perimeter (r = 0.261)), none of the correlation remained significant after adjustment for age and BMI; while AIS had significant correlations between serum total leptin and cortical vBMD (r = 0.362), cortical area (r = 0.367), and cortical thickness (r = 0.385) after the Hochberg-Benjamini procedure, however, none of the correlation remained significant after adjustment for age and BMI (Table 3). For sOB-R, controls had significant correlations with cortical vBMD (r = −0.229), cortical area (r = −0.248), and cortical thickness (r = −0.239) after the Hochberg-Benjamini procedure, and again none of the correlations remained significant after adjustment for age and BMI; while AIS had no significant correlation (Table 3).
Table 3. Correlations between cortical bone parameters, serum total leptin and sOB-R levels with and without age and BMI adjustment.
| Serum Total Leptin | sOB-R | ||||
| Controls | AIS | Controls | AIS | ||
| A)Volumetric BMD | |||||
| Cortical vBMD | r | 0.287** # | 0.362** # | −0.229* # | −0.116 |
| β | 0.054 | 0.042 | −0.144 | −0.800 | |
| B) Bone Morphometry | |||||
| Cortical Area | r | 0.294** # | 0.367** # | −0.248* # | −0.141 |
| β | 0.041 | 0.048 | −0.147 | −0.091 | |
| Cortical Thickness | r | 0.249* # | 0.385** # | −0.239* # | −0.134 |
| β | 0.028 | 0.081 | −0.158 | −0.083 | |
| Cortical Bone Perimeter | r | 0.261* # | −0.146 | −0.173 | −0.130 |
| β | 0.002 | −0.224 | −0.051 | −0.141 |
*correlation with p-value <0.05,
**correlation with p-value <0.01,
statistically significant after Hochberg-Benjamini correction for multiple comparison.
Abbreviations: AIS, adolescent idiopathic scoliosis; β, standardized coefficient calculated by multiple regression analysis with age and BMI adjustment; r, Pearson’s correlation coefficient; sOB-R, soluble leptin receptor; vBMD, volumetric bone mineral density.
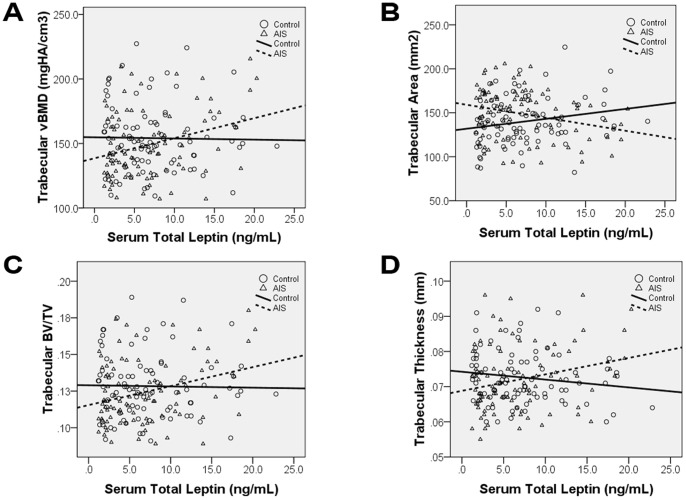
Results of correlation analysis with trabecular bone parameters were shown in Table 4. For serum total leptin, AIS showed distinctive correlation pattern that was absent in controls. With Pearson’s correlation analysis, AIS had significant correlations with trabecular vBMD (r = 0.303), trabecular area (r = −0.269), trabecular BV/TV (r = 0.303), and trabecular thickness (r = 0.254) after the Hochberg-Benjamini procedure, all of these correlations remained significant after age and BMI adjustment. In addition, trabecular number (β = 0.265) and trabecular separation (β = −0.262) became significant after age and BMI adjustment. Scatter plots of some of these correlations were shown in Figure 5 to illustrate the difference observed between AIS and control groups. It was evident that the AIS group showed increasing trend in the scatter plots with trabecular vBMD (A), trabecular BV/TV (C), trabecular thickness (D), and decreasing trend in trabecular area (B) versus serum total leptin. Controls, however, showed no correlation in the trabecular vBMD (A), trabecular BV/TV (C), and trabecular thickness (D) plots. For correlations between sOB-R and trabecular bone parameters, both AIS and controls showed no significant correlations (Table 4).
Table 4. Correlations between trabecular bone parameters, serum total leptin, and sOB-R levels with and without age and BMI adjustment.
| Serum Total Leptin | sOB-R | ||||
| Controls | AIS | Controls | AIS | ||
| A)Volumetric BMD | |||||
| Trabecular vBMD | r | −0.017 | 0.303** # | −0.085 | 0.171 |
| β | −0.007 | 0.378** # | −0.091 | 0.194 | |
| B) Bone Morphometry | |||||
| Trabecular Area | r | 0.206 | −0.269** # | −0.091 | −0.066 |
| β | 0.012 | −0.269* # | 0.009 | −0.090 | |
| C)Trabecular Bone Micro-architecture | |||||
| Trabecular BV/TV | r | −0.018 | 0.303** # | −0.084 | 0.172 |
| β | −0.010 | 0.377** # | −0.090 | 0.195 | |
| Trabecular Number | r | 0.101 | 0.148 | −0.133 | 0.179 |
| β | 0.137 | 0.265* # | −0.117 | 0.190 | |
| Trabecular Thickness | r | −0.150 | 0.254* # | 0.029 | 0.049 |
| β | −0.175 | 0.256* # | −0.001 | 0.066 | |
| Trabecular Separation | r | −0.095 | −0.163 | 0.116 | −0.162 |
| β | −0.149 | −0.262* # | 0.105 | −0.176 |
*correlation with p-value <0.05,
**correlation with p-value <0.01,
statistically significant after Hochberg-Benjamini correction for multiple comparison.
Abbreviations: AIS, adolescent idiopathic scoliosis; β, standardized coefficient calculated by multiple regression analysis with age and BMI adjustment; BV/TV, bone volume to tissue volume ratio; r, Pearson’s correlation coefficient; sOB-R, soluble leptin receptor; vBMD, volumetric bone mineral density.
Figure 5. Correlations of various HR-pQCT parameters with serum total leptin in AIS and controls.
Correlation analysis showed there was statistically significant correlation between serum total leptin and the following HR-pQCT parameters only in AIS: A) Trabecular volumetric bone mineral density (vBMD), B)Trabecular Area, C) Trabecular bone volume to tissue volume ratio (BV/TV), and D) Trabecular Thickness. The difference in correlations could be observed by crossing of the best fit lines.
Discussion
This is the first study investigating the correlation between serum total leptin, sOB-R and volumetric BMD, bone morphometry and trabecular bone micro-architecture parameters as assessed by HR-pQCT for AIS subjects.
AIS girls were found to have higher sOB-R level (Table 1). sOB-R is the main binding protein for leptin in the circulation [60], [61]. Transgenic mice with over-expression of sOB-R were found to have increased circulating total leptin and enhanced leptin signal [60], [62]. High sOB-R concentrations have been shown to neutralize leptin-mediated STAT3 signaling and anorexic responses in rats [63]. sOB-R might compete with membrane anchored leptin receptor and inhibit the transport of free leptin into cells and across the blood brain barrier, thus affecting the leptin bioavailability to the cells. Based on these studies, sOB-R acts in a complex manner in that, on one hand, it could prevent circulating leptin from clearance [60], [61] and may serve as a reservoir to maintain a constant pool of readily available leptin in lean subjects [60], [64], but on the other hand, cell and animal models suggested a predominant inhibitory effect of sOB-R on leptin signaling [65], [66], [67]. In the current study, AIS patients were shown to have 10% higher sOB-R level (p = 0.006; FDR-adjusted p = 0.060) and slightly lower serum total leptin level (p = 0.493), thus indicating a higher level of bound leptin and lower level of free leptin that could result in alteration of leptin bioavailability to the target tissues.
The correlation between BMI and circulating leptin level has been well documented, and was shown to be strong and positive [47], [68], [69], [70], [71], [72], [73]. The current study showed no significant difference in serum total leptin levels between AIS and control subjects, perhaps because the subjects’ range of BMI was lower and restricted in range as compared to previous study by Qiu et al. [56] that included girls with BMI over 23.0 kg/m2. Of note, these study findings of this study are similar to our previous study [15] when comparisons are made between the same subgroup of subjects with respect to BMI.
The negative correlation between serum total leptin and sOB-R found in normal controls of this study had also been reported in the literature [73]. This phenomenon suggests the presence of a negative feedback loop with which serum total leptin could affect and regulate sOB-R level [60], [74]. This feedback loop occurs in most hormonal systems to allow precise regulation of active hormone levels [61]. The absence of this correlation in AIS could suggest cellular dysregulation in sOB-R expression or a defect in the feedback loop, thus leading to persistently high level of sOB-R in AIS.
In addition to anthropometric abnormalities and abnormal leptin bioavailability, AIS girls also have low bone mass [4], [6], [9], [16], [75], [76], [77], [78]. Previous studies by our group and others have reported that osteopenia in AIS girls is systemic in nature and could affect the whole body including the spine, hip, distal radius, distal tibia, and calcaneus [4], [6], [11], [79], [80]. This systemic low bone mass is also likely to associate with lower bone strength and peak bone mass [11], [75], [81], which might contribute to curve progression [16], osteoporosis, osteoporotic fracture, and other associated complications in late adulthood [9]. As progression of scoliosis in osteoporotic spine has been reported in adults [82], the prevention or treatment of low bone mass in patients with AIS might prevent the progression of the scoliosis as well as minimize the long term complications of osteoporosis later in life. Unfortunately, what causes the manifestation of low bone mass in AIS girls is still unknown, and it is important to reveal the underlying causes before effective means of prevention or treatment can be formulated.
Previous studies on BMD in patients with AIS were limited by the two-dimensional projection measurement of DXA for a three-dimensional structure and the resolution of pQCT was not high enough to assess important bone quality parameters including trabecular bone micro-architecture. With recent advances in bone micro-imaging technology, HR-pQCT with a resolution of up to 82 µm is now available for separate analysis of bone morphometry and vBMD in the cortical and trabecular compartments together with detailed assessment of trabecular bone micro-architecture. This new technology allows more in-depth and differential studies on bone related AIS research.
When analyzed separately, AIS girls were found to have numerically lower bone mineral status in all bone compartments with marginally significant difference detected for average vBMD and cortical vBMD. The % differences in all bone compartments were in general agreement with previous studies which reported that AIS girls had average areal BMD values 4.5% lower than controls [4], [9]. In particular, the numerically lower average vBMD found in this study was in agreement with those reported by Lee et al. [78] and Cheung et al. [6] showing lower integrated vBMD of the cortical and trabecular bones at the non-dominant distal radius and distal tibia of AIS girls. Likely due to the notably higher variance in measurement of vBMD of the metabolically active trabecular compartment [83], the difference in trabecular vBMD was not statistically significant although the % difference was similar to that of cortical vBMD (Table 2).
Leptin appears to affect bone metabolism through both central and peripheral pathways with different catabolic and anabolic effects on bone [31], [39], [84], [85]. It was suggested that there might be a balance between these two seemingly opposite effects of leptin on bone [86]. The differences in leptin bioavailability found in AIS girls versus controls (Table 1) might correspondingly affect the central and peripheral tissues differently. As suggested by a number of previous studies, the effects of leptin mediated by the CNS seemed to affect primarily cortical bone. In mice, leptin seems to regulate cortical bone formation through sympathetic activation [84], and ob/ob mice have diminished sympathetic tone [40]. Furthermore, β1- and β2-adrenergic receptor knock-out mice show decreased femoral mass and cortical thickness when compared to wild type mice [84]. Data from mice studies also indicate that leptin may also mediate cortical bone formation by regulating the expression of several neuropeptides in the hypothalamus. Mice lacking the neuropeptide Y receptor Y2 were shown to have increased cortical bone mass and density in the femora [87], indicating that neuropeptide Y inhibits cortical bone formation [84]. However, leptin has also been shown to increase expression of neuromedin U [88], which may decrease both cortical and trabecular bone [89]. The findings from these mice studies might serve to explain why marginal statistical significance on the difference was mainly detected for cortical vBMD but not for trabecular vBMD (Table 2).
Although the difference did not reach statistical significance for the trabecular bone micro-architecture parameters, AIS girls had numerically lower trabecular BV/TV, trabecular number, trabecular thickness, and higher trabecular separation when compared with controls. This was in agreement with findings by Lam et al. [79] who noted deranged bone quality as evidenced by lower Quantitative Ultrasound measurements at the non-dominant calcaneus of AIS subjects when compared with controls.
The change in bone quality in AIS is an important endophenotype. It is important to evaluate the correlation pattern of serum total leptin level and sOB-R with bone parameters which was found to be distinct in AIS subjects when compared with controls. On one hand, in girls with AIS there was a significant correlation between serum total leptin and all trabecular bone parameters (Table 4). The correlations became stronger after the adjustment for age and BMI, suggesting that the response to leptin in the trabecular bone of AIS girls is independent of age and BMI. However, on the other hand, the significant correlations between serum total leptin, sOB-R and cortical parameters became insignificant after age and BMI adjustment, suggested that the effects of age and BMI in cortical bone are much stronger than leptin and sOB-R (Table 3). It might be that the response of bone tissue to altered leptin signaling is not uniform between cortical and trabecular bone throughout the skeleton [90]. The significant correlation between serum total leptin and the trabecular parameters that was seen in AIS was not apparent in controls, thus potentially indicating an abnormal response to leptin or leptin signaling in the trabecular compartment in AIS. Trabecular bone has a much larger surface to volume ratio and is metabolically more active when compared with cortical bone [83]. This, when coupled with our findings that the trabecular area in AIS was greater than controls, the statistically significant correlation between serum total leptin level and the trabecular parameters might be expected.
The evidence of leptin effects on bone metabolism remain controversial, and together with the added uncertainty of abnormal leptin bioavailability under diseased status, careful consideration is needed during the interpretation of the discrepancy in correlation pattern of serum total leptin/sOB-R levels with bone quality parameters in our study. The possible effects of the abnormal leptin bioavailability on cells and bone metabolism are speculations based on previous findings. A scoliotic animal model would be required to provide causal evidences between abnormal leptin bioavailability/sensitivity and AIS. As mentioned earlier, AIS was associated with low bone mass which was correlated with curve severity and has been shown to be a significant prognostic factor for curve progression in AIS [16], [78]. The role of leptin and sOB-R on bone metabolism could potentially be the key for better understanding the origin of low bone mass in AIS. Research in this area can potentially lead to novel preventive or therapeutic measures for treating low bone mass and related curve progression in AIS subjects.
In summary, this is an exploratory study comparing the relationship between serum total leptin, sOB-R and cortical and trabecular vBMDs, bone morphometry and trabecular bone micro-architecture in young girls affected by AIS and normal controls. Correlation pattern between HR-pQCT bone parameters and serum total leptin and sOB-R were distinctly different between AIS and control subjects. The implication of these findings, whether there was increased response of trabecular bone to changes in the biochemical milieu in AIS and/or whether altered leptin bioavailability plays a role in the etiopathogenesis of AIS and its accompanying osteopenia warrant further studies.
Acknowledgments
The authors would like to acknowledge Ms Annie Yim, Ms Fish Yu, Ms Queenie Mak, Ms Tracy Wong, Ms Christy Chan, and Dr. Benson Yeung for their help with subject recruitments and technical support.
Funding Statement
Research grants were received from Fondation Yves Cotrel - Institut de France, RGC of Hong Kong SAR (Project no: 467808 and 468809), and CUHK Direct Grant (2010.2.042). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA (2008) Adolescent idiopathic scoliosis. Lancet 371: 1527–1537. [DOI] [PubMed] [Google Scholar]
- 2. Luk K, Lee C, Cheung K, Cheng J, Ng B, et al. (2010) Clinical Effectiveness of School Screening for Adolescent Idiopathic Scoliosis: A Large Population-Based Retrospective Cohort Study. Spine 35: 1607–1614. [DOI] [PubMed] [Google Scholar]
- 3. Cheung CSK, Lee WTK, Tse YK, Tang SP, Lee KM, et al. (2003) Abnormal Peri-pubertal Anthropometric Measurements and Growth Pattern in Adolescent Idiopathic Scoliosis – a Study of 598 Patients. Spine 28: 2152–2157. [DOI] [PubMed] [Google Scholar]
- 4. Cheng JCY, Qin L, Cheung CSK, Sher AHL, Lee KM, et al. (2000) Generalized Low Areal and Volumetric Bone Mineral Density in Adolescent Idiopathic Scoliosis. Journal of Bone and Mineral Research 15: 1587–1595. [DOI] [PubMed] [Google Scholar]
- 5.Wang WJ, Hung VW, Lam TP, Ng BK, Qin L, et al.. (2010) The association of disproportionate skeletal growth and abnormal radius dimension ratio with curve severity in adolescent idiopathic scoliosis. European Spine Journal. [DOI] [PMC free article] [PubMed]
- 6. Cheung CSK, Lee WTK, Tse YK, Guo X, Qin L, et al. (2006) Generalized osteopenia in Adolescent Idiopathic Scoliosis - Association with abnormal pubertal growth, bone turnover, and calcium intake? Spine 31: 330–338. [DOI] [PubMed] [Google Scholar]
- 7. Cheng JCY, Guo X (1997) Osteoporosis in Adolescent Idiopathic Scoliosis – A Primary Problem or Secondary to the Spinal Deformity? Spine 22: 1716–1721. [DOI] [PubMed] [Google Scholar]
- 8. Archer IA, Dickson RA (1985) Stature and idiopathic scoliosis. A prospective study. J Bone Joint Surg Br 67: 185–188. [DOI] [PubMed] [Google Scholar]
- 9. Cheng JCY, Guo X, Sher AH (1999) Persistent osteopenia in adolescent idiopathic scoliosis. A longitudinal follow up study. Spine (Phila Pa 1976) 24: 1218–1222. [DOI] [PubMed] [Google Scholar]
- 10.Buric M, Momcilovic B (1982) Growth pattern and skeletal age in school girls with idiopathic scoliosis. Clin Orthop Relat Res: 238–242. [PubMed]
- 11. Cook SD, Harding AF, Morgan EL, Nicholson RJ, Thomas KA, et al. (1987) Trabecular bone mineral density in idiopathic scoliosis. J Pediatr Orthop 7: 168–174. [DOI] [PubMed] [Google Scholar]
- 12.Nordwall A, Willner S (1975) A study of skeletal age and height in girls with idiopathic scoliosis. Clin Orthop Relat Res: 6–10. [DOI] [PubMed]
- 13.Willner S (1974) A study of growth in girls with adolescent idiopathic structural scoliosis. Clin Orthop Relat Res: 129–135. [PubMed]
- 14. Willner S (1974) Growth in height of children with scoliosis. Acta Orthop Scand 45: 854–866. [DOI] [PubMed] [Google Scholar]
- 15. Liu Z, Tam EMS, Sun GQ, Lam TP, Zhu ZZ, et al. (2012) Abnormal Leptin Bioavailability in Adolescent Idiopathic Scoliosis - an Important New Finding. Spine (Phila Pa 1976) 37: 599–604. [DOI] [PubMed] [Google Scholar]
- 16. Hung VWY, Qin L, Cheung CSK, Lam TP, Ng BKW, et al. (2005) Osteopenia: a new prognostic factor of curve progression in adolescent idiopathic scoliosis. THe Journal of Bone and Joint Surgery 87: 2709–2716. [DOI] [PubMed] [Google Scholar]
- 17. Beltowski J (2012) Leptin and the regulation of endothelial function in physiological and pathological conditions. Clin Exp Pharmacol Physiol 39: 168–178. [DOI] [PubMed] [Google Scholar]
- 18. Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, et al. (1995) Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269: 540–543. [DOI] [PubMed] [Google Scholar]
- 19. Dardeno TA, Chou SH, Moon HS, Chamberland JP, Fiorenza CG, et al. (2010) Leptin in human physiology and therapeutics. Front Neuroendocrinol 31: 377–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, et al. (2011) Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab 301: E567–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelesidis T, Kelesidis I, Chou S, Mantzoros CS (2010) Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med 152: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fruhbeck G (2006) Intracellular signalling pathways activated by leptin. Biochem J 393: 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, et al. (1995) Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269: 543–546. [DOI] [PubMed] [Google Scholar]
- 24. Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, et al. (1995) Identification and expression cloning of a leptin receptor, OB-R. Cell 83: 1263–1271. [DOI] [PubMed] [Google Scholar]
- 25. Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, et al. (1996) Abnormal splicing of the leptin receptor in diabetic mice. Nature 379: 632–635. [DOI] [PubMed] [Google Scholar]
- 26. Cioffi JA, Shafer AW, Zupancic TJ, Smith-Gbur J, Mikhail A, et al. (1996) Novel B219/OB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nat Med 2: 585–589. [DOI] [PubMed] [Google Scholar]
- 27. Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, et al. (1996) A role for leptin and its cognate receptor in hematopoiesis. Curr Biol 6: 1170–1180. [DOI] [PubMed] [Google Scholar]
- 28. Hess R, Pino AM, Rios S, Fernandez M, Rodriguez JP (2005) High affinity leptin receptors are present in human mesenchymal stem cells (MSCs) derived from control and osteoporotic donors. J Cell Biochem 94: 50–57. [DOI] [PubMed] [Google Scholar]
- 29. Reseland JE, Syversen U, Bakke I, Qvigstad G, Eide LG, et al. (2001) Leptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralization. J Bone Miner Res 16: 1426–1433. [DOI] [PubMed] [Google Scholar]
- 30. Zhang F, Chen Y, Heiman M, Dimarchi R (2005) Leptin: structure, function and biology. Vitam Horm 71: 345–372. [DOI] [PubMed] [Google Scholar]
- 31. Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG (2000) Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept 92: 73–78. [DOI] [PubMed] [Google Scholar]
- 32. Gordeladze JO, Drevon CA, Syversen U, Reseland JE (2002) Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: Impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem 85: 825–836. [DOI] [PubMed] [Google Scholar]
- 33. Maor G, Rochwerger M, Segev Y, Phillip M (2002) Leptin acts as a growth factor on the chondrocytes of skeletal growth centers. J Bone Miner Res 17: 1034–1043. [DOI] [PubMed] [Google Scholar]
- 34. Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, et al. (1999) Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology 140: 1630–1638. [DOI] [PubMed] [Google Scholar]
- 35. Handschin AE, Trentz OA, Hemmi S, Wedler V, Trentz O, et al. (2007) Leptin increases extracellular matrix mineralization of human osteoblasts from heterotopic ossification and normal bone. Ann Plast Surg 59: 329–333. [DOI] [PubMed] [Google Scholar]
- 36. Lamghari M, Tavares L, Camboa N, Barbosa MA (2006) Leptin effect on RANKL and OPG expression in MC3T3-E1 osteoblasts. J Cell Biochem 98: 1123–1129. [DOI] [PubMed] [Google Scholar]
- 37. Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge JM, et al. (2002) Leptin inhibits osteoclast generation. J Bone Miner Res 17: 200–209. [DOI] [PubMed] [Google Scholar]
- 38. Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, et al. (2004) Serum leptin level is a regulator of bone mass. Proceedings of the National Academy of Sciences of the United States of America 101: 3258–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, et al. (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100: 197–207. [DOI] [PubMed] [Google Scholar]
- 40. Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, et al. (2002) Leptin regulates bone formation via the sympathetic nervous system. Cell 111: 305–317. [DOI] [PubMed] [Google Scholar]
- 41. Ducy P, Schinke T, Karsenty G (2000) The osteoblast: a sophisticated fibroblast under central surveillance. Science 289: 1501–1504. [DOI] [PubMed] [Google Scholar]
- 42. Matkovic V, Ilich JZ, Skugor M, Badenhop NE, Goel P, et al. (1997) Leptin is inversely related to age at menarche in human females. J Clin Endocrinol Metab 82: 3239–3245. [DOI] [PubMed] [Google Scholar]
- 43. Quinton ND, Smith RF, Clayton PE, Gill MS, Shalet S, et al. (1999) Leptin binding activity changes with age: the link between leptin and puberty. Journal of Clinical Endocrinology and Metabolism 84: 2336–2341. [DOI] [PubMed] [Google Scholar]
- 44. Grivas TB, Burwell RG, Mihas C, Vasiliadis ES, Triantafyllopoulos G, et al. (2009) Relatively lower body mass index is associated with an excess of severe truncal asymmetry in healthy adolescents: Do white adipose tissue, leptin, hypothalamus and sympathetic nervous system influence truncal growth asymmetry? Scoliosis 4: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu T, Sun X, Zhu Z, Zheng X, Qian B, et al. (2012) Role of high central leptin activity in a scoliosis model created in bipedal amputated mice. Stud Health Technol Inform 176: 31–35. [PubMed] [Google Scholar]
- 46. Qiu Y, Sun X, Qiu X, Li W, Zhu Z, et al. (2007) Decreased circulating leptin level and its association with body and bone mass in girls with adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 32: 2703–2710. [DOI] [PubMed] [Google Scholar]
- 47. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, et al. (1996) Serum immunoreactive-leptin concentrations in normal-weight and obese humans. New England Journal of Medicine 334: 292–295. [DOI] [PubMed] [Google Scholar]
- 48. Hassink SG, Sheslow DV, de Lancey E, Opentanova I, Considine RV, et al. (1996) Serum leptin in children with obesity: relationship to gender and development. Pediatrics 98: 201–203. [PubMed] [Google Scholar]
- 49. Lenke LG, Betz RR, Harms J, Bridwell KH, Clements DH, et al. (2001) Adolescent idiopathic scoliosis. A new classification to determine extent of spinal arthrodesis. Journal of Bone and Joint Surgery - Series A 83: 1169–1181. [PubMed] [Google Scholar]
- 50. Kono K, Asazuma T, Suzuki N, Ono T (2000) Body height correction in scoliosis patients for pulmonary function test. J Orthop Surg (Hong Kong) 8: 19–26. [DOI] [PubMed] [Google Scholar]
- 51. Ylikoski M (2003) Height of girls with adolescent idiopathic scoliosis. European Spine Journal 12: 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheng JCY, Leung SSF, Lau J (1996) Anthropometric Measurements and Body Proportions Among Chinese Children. Clinical Orthopaedics and Related Research 323: 22–30. [DOI] [PubMed] [Google Scholar]
- 53. Kirmani S, Christen D, van Lenthe GH, Fischer PR, Bouxsein ML, et al. (2009) Bone structure at the distal radius during adolescent growth. J Bone Miner Res 24: 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu XS, Cohen A, Shane E, Yin PT, Stein EM, et al. (2010) Bone density, geometry, microstructure, and stiffness: Relationships between peripheral and central skeletal sites assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J Bone Miner Res 25: 2229–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Laib A, Hauselmann HJ, Ruegsegger P (1998) In vivo high resolution 3D-QCT of the human forearm. Technol Health Care 6: 329–337. [PubMed] [Google Scholar]
- 56. MacNeil JA, Boyd SK (2007) Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys 29: 1096–1105. [DOI] [PubMed] [Google Scholar]
- 57. Laib A, Hildebrand T, Hauselmann HJ, Ruegsegger P (1997) Ridge number density: a new parameter for in vivo bone structure analysis. Bone 21: 541–546. [DOI] [PubMed] [Google Scholar]
- 58. Hildebrand T, Rüegsegger P (1997) A new method for the model-independent assessment of thickness in three-dimensional images. Journal of Microscopy 185: 67–75. [Google Scholar]
- 59. Laib A, Ruegsegger P (1999) Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone 24: 35–39. [DOI] [PubMed] [Google Scholar]
- 60. Huang L, Wang Z, Li C (2001) Modulation of circulating leptin levels by its soluble receptor. J Biol Chem 276: 6343–6349. [DOI] [PubMed] [Google Scholar]
- 61. Chan JL, Bluher S, Yiannakouris N, Suchard MA, Kratzsch J, et al. (2002) Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes 51: 2105–2112. [DOI] [PubMed] [Google Scholar]
- 62.Lou PH, Yang G, Huang L, Cui Y, Pourbahrami T, et al.. (2010) Reduced body weight and increased energy expenditure in transgenic mice over-expressing soluble leptin receptor. PLoS One. [DOI] [PMC free article] [PubMed]
- 63. Zhang J, Scarpace PJ (2009) The soluble leptin receptor neutralizes leptin-mediated STAT3 signalling and anorexic responses in vivo. Br J Pharmacol 158: 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gavrilova O, Barr V, Marcus-Samuels B, Reitman M (1997) Hyperleptinemia of pregnancy associated with the appearance of a circulating form of the leptin receptor. Journal of Biological Chemistry 272: 30546–30551. [DOI] [PubMed] [Google Scholar]
- 65. Yang G, Ge H, Boucher A, Yu X, Li C (2004) Modulation of direct leptin signaling by soluble leptin receptor. Mol Endocrinol 18: 1354–1362. [DOI] [PubMed] [Google Scholar]
- 66. Zastrow O, Seidel B, Kiess W, Thiery J, Keller E, et al. (2003) The soluble leptin receptor is crucial for leptin action: evidence from clinical and experimental data. Int J Obes Relat Metab Disord 27: 1472–1478. [DOI] [PubMed] [Google Scholar]
- 67. Tu H, Kastin AJ, Hsuchou H, Pan W (2008) Soluble receptor inhibits leptin transport. J Cell Physiol 214: 301–305. [DOI] [PubMed] [Google Scholar]
- 68. Yannakoulia M, Yiannakouris N, Bluher S, Matalas AL, Klimis-Zacas D, et al. (2003) Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. J Clin Endocrinol Metab 88: 1730–1736. [DOI] [PubMed] [Google Scholar]
- 69. Misra M, Miller KK, Almazan C, Ramaswamy K, Aggarwal A, et al. (2004) Hormonal and body composition predictors of soluble leptin receptor, leptin, and free leptin index in adolescent girls with anorexia nervosa and controls and relation to insulin sensitivity. J Clin Endocrinol Metab 89: 3486–3495. [DOI] [PubMed] [Google Scholar]
- 70. Mann DR, Johnson AO, Gimpel T, Castracane VD (2003) Changes in circulating leptin, leptin receptor, and gonadal hormones from infancy until advanced age in humans. J Clin Endocrinol Metab 88: 3339–3345. [DOI] [PubMed] [Google Scholar]
- 71. Malincikova J, Stejskal D, Hrebicek J (2000) Serum leptin and leptin receptors in healthy prepubertal children: relations to insulin resistance and lipid parameters, body mass index (BMI), tumor necrosis factor alpha (TNF alpha), heart fatty acid binding protein (hFABP), and IgG anticardiolipin (ACL-IgG). Acta Univ Palacki Olomuc Fac Med 143: 51–57. [DOI] [PubMed] [Google Scholar]
- 72. Celi F, Galmacci G, Bini V, Papi F, Contessa G, et al. (2005) BMI-conditional standard of leptin serum values in children: proposal of a statistical procedure for the analysis of influencing variables. J Pediatr Endocrinol Metab 18: 1399–1408. [DOI] [PubMed] [Google Scholar]
- 73. Kratzsch J, Lammert A, Bottner A, Seidel B, Mueller G, et al. (2002) Circulating soluble leptin receptor and free leptin index during childhood, puberty, and adolescence. Journal of Clinical Endocrinology and Metabolism 87: 4587–4594. [DOI] [PubMed] [Google Scholar]
- 74. Schaab M, Kausch H, Klammt J, Nowicki M, Anderegg U, et al. (2012) Novel regulatory mechanisms for generation of the soluble leptin receptor: implications for leptin action. PLoS One 7: e34787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cheng JC, Hung VW, Lee WT, Yeung HY, Lam TP, et al. (2006) Persistent osteopenia in adolescent idiopathic scoliosis - longitudinal monitoring of bone mineral density until skeletal maturity. Stud Health Technol Inform 123: 47–51. [PubMed] [Google Scholar]
- 76. Yeung HY, Qin L, Hung VW, Lee KM, Guo X, et al. (2006) Lower degree of mineralization found in cortical bone of adolescent idiopathic scoliosis (AIS). Stud Health Technol Inform 123: 599–604. [PubMed] [Google Scholar]
- 77. Snyder BD, Katz DA, Myers ER, Breitenbach MA, Emans JB (2005) Bone density accumulation is not affected by brace treatment of idiopathic scoliosis in adolescent girls. J Pediatr Orthop 25: 423–428. [DOI] [PubMed] [Google Scholar]
- 78. Lee WTK, Cheung CSK, Tse YK, Guo X, Qin L, et al. (2005) Generalized low bone mass of girls with adolescent idiopathic scoliosis is related to inadequate calcium intake and weight bearing physical activity in peripubertal period. Osteoporos Int 16: 1024–1035. [DOI] [PubMed] [Google Scholar]
- 79. Lam TP, Hung VW, Yeung HY, Tse YK, Chu WC, et al. (2011) Abnormal bone quality in adolescent idiopathic scoliosis: a case-control study on 635 subjects and 269 normal controls with bone densitometry and quantitative ultrasound. Spine (Phila Pa 1976) 36: 1211–1217. [DOI] [PubMed] [Google Scholar]
- 80. Li XF, Li H, Liu ZD, Dai LY (2008) Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J 17: 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Velis KP, Healey JH, Schneider R (1989) Peak skeletal mass assessment in young adults with idiopathic scoliosis. Spine (Phila Pa 1976) 14: 706–711. [DOI] [PubMed] [Google Scholar]
- 82. Saggese G, Baroncelli GI, Bertelloni S (2001) Osteoporosis in children and adolescents: diagnosis, risk factors, and prevention. J Pediatr Endocrinol Metab 14: 833–859. [DOI] [PubMed] [Google Scholar]
- 83.Dempster DW (1992) Bone Remodeling. In: Coe FL, Favus MJ, editors. Disorders of bone and mineral metabolism. New York: Raven Press. 355–380.
- 84. Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, et al. (2005) Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res 20: 994–1001. [DOI] [PubMed] [Google Scholar]
- 85. Lorentzon R, Alehagen U, Boquist L (1986) Osteopenia in mice with genetic diabetes. Diabetes Res Clin Pract 2: 157–163. [DOI] [PubMed] [Google Scholar]
- 86. Thomas T (2003) Leptin: a potential mediator for protective effects of fat mass on bone tissue. Joint Bone Spine 70: 18–21. [DOI] [PubMed] [Google Scholar]
- 87. Baldock PA, Allison S, McDonald MM, Sainsbury A, Enriquez RF, et al. (2006) Hypothalamic regulation of cortical bone mass: opposing activity of Y2 receptor and leptin pathways. J Bone Miner Res 21: 1600–1607. [DOI] [PubMed] [Google Scholar]
- 88. Wren AM, Small CJ, Abbott CR, Jethwa PH, Kennedy AR, et al. (2002) Hypothalamic actions of neuromedin U. Endocrinology. 143: 4227–4234. [DOI] [PubMed] [Google Scholar]
- 89. Sato S, Hanada R, Kimura A, Abe T, Matsumoto T, et al. (2007) Central control of bone remodeling by neuromedin U. Nat Med. 13: 1234–1240. [DOI] [PubMed] [Google Scholar]
- 90. Hamrick MW, Pennington C, Newton D, Xie D, Isales C (2004) Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 34: 376–383. [DOI] [PubMed] [Google Scholar]