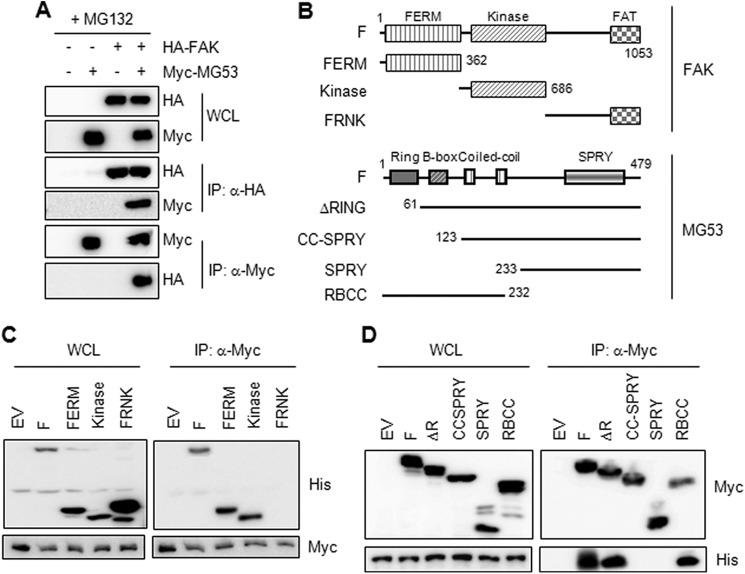
FIGURE 3.
The B-box of MG53 is a binding domain for FAK. A, different combinations of Myc-MG53 (0.5 μg) and HA-FAK (1.5 μg) were cotransfected into HEK 293 cells for 36 h. The cells were further treated with MG132 (5 μm) for 12 h, and then the molecular association of MG53 with FAK was monitored by immunoprecipitation (IP) with anti-HA and anti-Myc antibodies. WCL, whole cell lysate. B, FAK and MG53 deletion constructs. F, full-length; FERM, 4.1 protein/ezrin/radixin/moesin; FAT, focal adhesion targeting domain; FRNK, FAK-related non-kinase domain; CC, coiled-coil domain; SPRY, SPla and RYanodine receptor domain; RBCC, RING, B-box, and coiled-coil domains. C and D, HEK 293 cells were cotransfected with Myc-MG53 (0.5 μg) and various His-FAK mutants (1.5 μg) or with His-FAK (1.5 μg) and various Myc-MG53 mutants (0.5 μg) for 48 h and subsequently treated with MG132 (5 μm) for 12 h. The molecular association of MG53 with FAK was determined by immunoprecipitation (IP). EV, empty vector.