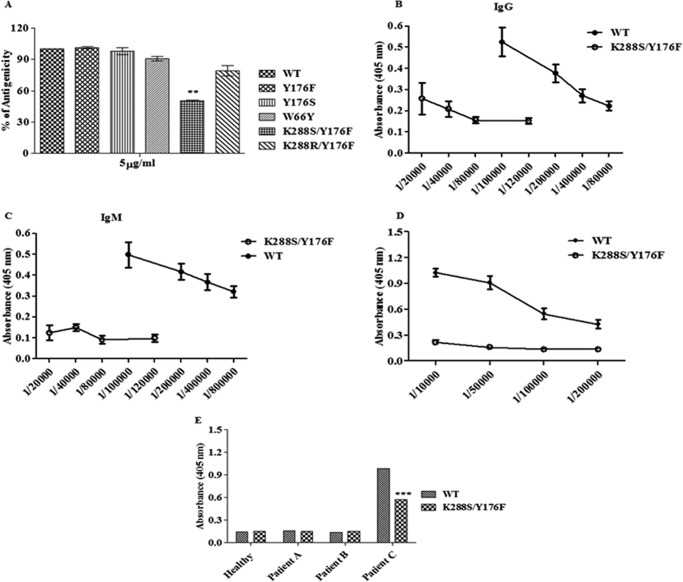
FIGURE 4.
Indirect ELISA of wild-type EcA and variants. A, microtiter plates were coated with wild-type EcA and variants (5 μg/ml), probed with primary (rabbit anti-EcA), and developed by secondary (HRP-conjugated polyclonal goat anti-rabbit IgG) antibodies by the indirect ELISA method. The experiment was performed three times. B and C, immunogenicity study in mice. Microtiter plates were coated with wild-type EcA and K288S/Y176F variant (5 μg/ml). Different dilutions of serum (obtained on day 74 after immunization) from mice immunized with wild-type EcA and K288S/Y176F was prepared and added to the wells in duplicate. Primary antibodies (goat anti-mouse IgM or anti-mouse IgG) and secondary antibody (HRP-conjugated polyclonal rabbit anti-goat Ig) were used. The titer of IgG (B) and IgM (C) antibodies were determined by the indirect ELISA method. D, microtiter plates were coated with wild-type EcA and K288S/Y176F (5 μg/ml). Different dilutions of serum obtained from mice immunized with wild-type EcA were added to the wells. Binding of serum IgG antibody to wild-type EcA and K288S/Y176F was determined as described above. Immunization studies were repeated two times. E, microtiter plates were coated with wild-type EcA and K288S/Y176F. Appropriate dilution of serum obtained from a healthy individual, an ALL patient without asparaginase therapy (patient A), and ALL patients who were given asparaginase therapy for 5 days (patient B) and 45 days (patient C) were added to the wells. The data shown here are representative of three (n = 3) patients from each group. The binding of serum IgG with wild-type EcA and K288S/Y176F was determined as described above. Mean ± S.D. are shown. ***, p < 0.001.