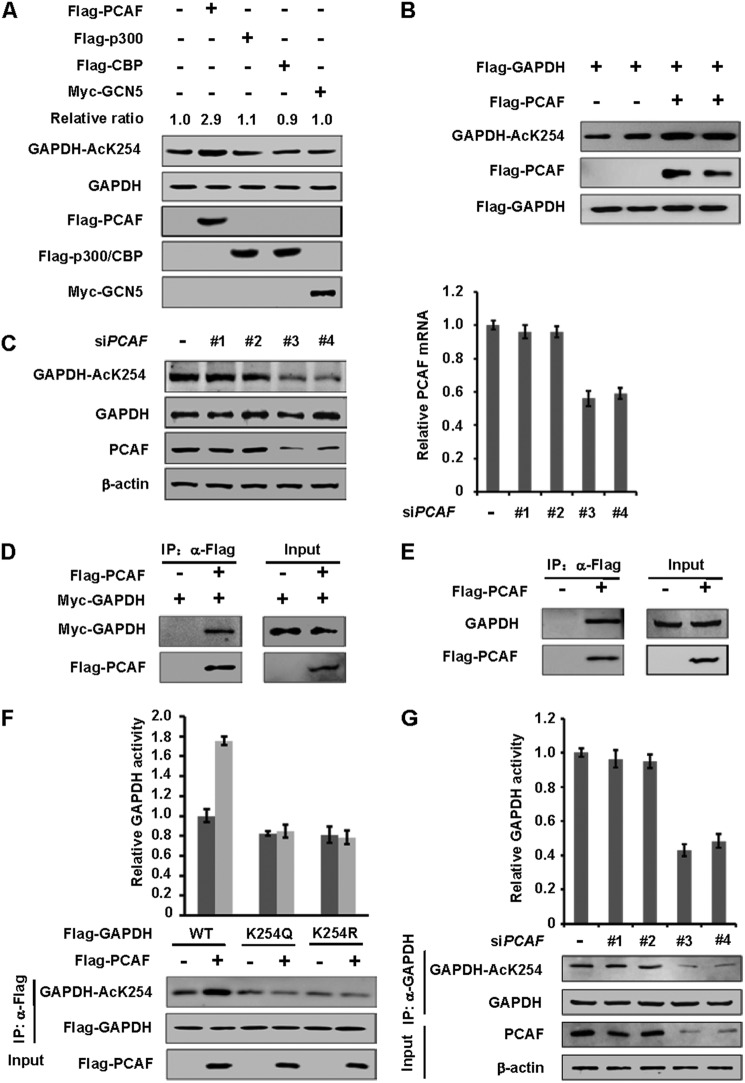
FIGURE 3.
PCAF is one of the acetyltransferases of GAPDH. A, overexpression of PCAF, but not other acetyltransferases, increases endogenous GAPDH K254 acetylation level. Indicated plasmids were expressed in HEK 293T cells. Endogenous acetylation levels of GAPDH were determined by anti-AcGAPDH (K254) antibody. Relative ratios of GAPDH K254 acetylation were calculated from normalizing against GAPDH. B, overexpression of PCAF increases GAPDH K254 acetylation levels. The indicated plasmids were expressed in HEK 293T cells, and the acetylation and protein levels were analyzed by Western blot. C, PCAF knockdown decreases endogenous GAPDH acetylation at K254. A549 cells were transfected with siPCAF (four different oligos) or negative control (siNC) as previously described, and the acetylation and protein levels were analyzed by Western blot (left panel). PCAF transcription level was measured by qPCR (right panel). D, exogenous GAPDH interacts with PCAF. The indicated plasmids were expressed in HEK 293T cells and proteins were affinity purified. GAPDH and PCAF protein levels were determined against indicated antibodies, respectively. E, endogenous GAPDH interacts with PCAF. Flag-PCAF was expressed in HEK 293T cells and proteins were affinity purified. GAPDH and PCAF protein levels were determined against indicated antibodies, respectively. F, PCAF increases the enzyme activity of WT, but not mutants of GAPDH. The indicated plasmids were expressed in HEK 293T cells and affinity purified. Different samples were analyzed by Western blot and enzyme activities were measured as described in Fig. 2A. Mean values of relative enzyme activity of triplicate experiments ±S.D. are presented. G, PCAF knockdown decreases endogenous GAPDH activity. HEK 293T cells were transfected with siPCAF or negative control (siNC) as previously described and endogenous GAPDH were purified. Different samples were analyzed by Western blot and enzyme activities were measured as described in Fig. 2A. Mean values of relative enzyme activity of triplicate experiments ±S.D. are presented.